Mobile medication manager application to improve adherence with immunosuppressive therapy in renal transplant recipients: A randomized controlled trial
- PMID: 31689320
- PMCID: PMC6830819
- DOI: 10.1371/journal.pone.0224595
Mobile medication manager application to improve adherence with immunosuppressive therapy in renal transplant recipients: A randomized controlled trial
Abstract
Background: Nonadherence to immunosuppressive therapy after renal transplantation is associated with poor graft outcomes. We aimed to evaluate whether the use of the Adhere4U mobile medication manager application could improve adherence among renal transplant recipients ≥1 year posttransplantation. Adhere4U can provide medication reminders, monitor medication use, and provide information on immunosuppressants.
Methods: We conducted a prospective randomized controlled study to compare the rate of nonadherence to index immunosuppressant (tacrolimus or cyclosporine) in a group using the Adhere4U app (mobile group) and in another group receiving conventional care (control group). The primary outcome was the nonadherence rate, which was evaluated using an electronic medication event monitoring system during the 6-month intervention period. Our secondary outcome included self-reported adherence using the Basel Assessment of Adherence to Immunosuppressive Medication Scale (BAASIS) and the visual analog scale (VAS) based on a 4-week recall on days 28, 90, and 180. Longitudinal data of repeated measures of self-rated adherence were analyzed using generalized estimating equations (GEE) to compare the between-group difference in adherence change over time.
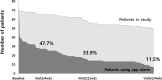
Results: Between November 2013 and May 2015, 138 renal transplant recipients were randomly allocated to the control (n = 67) or the mobile group (n = 71). The overall nonadherence rate over the 6-month study period by electronic monitoring was 63.6%, with no between-group difference [mobile group, 65.0% (n = 39/60); control group, 62.1% (n = 36/58); odds ratio 1.14; 95% confidence interval 0.53-2.40; p = 0.89]. Self-rated nonadherence assessed using the BAASIS and VAS at baseline was 53.7% and 51.5%, respectively. Although the self-rated nonadherence by BAASIS of the mobile group was lower than the control group throughout the study period, there was no between-group difference in the change of nonadherence over time (χ2 = 2.82, df = 3, p = 0.42 by logistic GEE). There also was no significant between-group difference in the nonadherence by VAS (χ2 = 1.71, df = 3, p = 0.63 by logistic GEE) over time. The main limitation of this study was the low rate of patient engagement with the app among the mobile group. The rate of app use was 47.6% (31/65) at 28 days, 33.9% (19/56) at 90 days, and 11.5% (6/52) at 180 days.
Conclusions: The Adhere4U application did not improve adherence to immunosuppressive therapy. Our evidence is limited by the high rate of attrition. Further studies on strategies to facilitate patient engagement with mobile interventions are warranted.
Conflict of interest statement
The authors have no conflicts of interest to declare.Funding provided by Astellas Pharma Korea, Inc. does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Prihodova L, Nagyova I, Rosenberger J, Majernikova M, Roland R, Groothoff JW, et al. Adherence in patients in the first year after kidney transplantation and its impact on graft loss and mortality: a cross-sectional and prospective study. J Adv Nurs. 2014;70: 2871–2883. 10.1111/jan.12447 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous