Plasma Androgen Receptor in Prostate Cancer
- PMID: 31689899
- PMCID: PMC6896184
- DOI: 10.3390/cancers11111719
Plasma Androgen Receptor in Prostate Cancer
Abstract
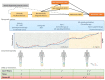
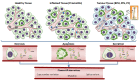
The therapeutic landscape of prostate cancer has expanded rapidly over the past 10 years, and there is now an even greater need to understand the biological mechanisms of resistance and to develop noninvasive biomarkers to guide treatment. The androgen receptor (AR) is known to be involved in the pathogenesis and progression of prostate cancer. Recently, highly sensitive next-generation sequencing and PCR-based methods for analyzing androgen receptor gene (AR) copy numbers (CN) and mutations in plasma were established in cell-free DNA (cfDNA) of patients with castration-resistant prostate cancer (CRPC) treated with different drugs. The study of cfDNA holds great promise for improving treatment in CRPC, especially in the advanced stage of the disease. Recent findings showed the significant association of plasma AR aberrations with clinical outcome in CRPC patients treated with AR-directed therapies, whereas no association was observed in patients treated with taxanes. This suggests the potential for using plasma AR as a biomarker for selecting treatment, i.e., hormone therapy or chemotherapy, and the possibility of modulating taxane dose. In recent years, plasma AR status has also been investigated in association with novel agents, such as 177Lu-PSMA radioligand therapy and PARP inhibitors. This review will focus on AR testing in plasma that may have clinical utility for treatment selection in advanced prostate cancer.
Keywords: androgen receptor; biomarkers; plasma DNA; prostate cancer.
Conflict of interest statement
V.C. has received speaker honoraria or travel support from Astellas, Janssen-Cilag, and Sanofi-Aventis, and has received consulting fee from Bayer. U.D.G. has served as consultant/advisory board member for Astellas, Bayer, BMS, Ipsen, Janssen, Merck, Pfizer, Sanofi, and has received travel support from BMS, Ipsen, Janssen, Pfizer, and has received research funding from AstraZeneca, Roche, Sanofi (Inst). No potential conflicts of interest were disclosed by the other authors.
Figures
References
-
- Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A., Eisenberger M.A., Higano C., Bubley G.J., Dreicer R., et al. Design and endpoints of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the prostate cancer clinical trials working group. J. Clin. Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. - DOI - PMC - PubMed
-
- Scher H.I., Morris M.J., Stadler W.M., Higano C., Basch E., Fizazi K., Antonarakis E.S., Beer T.M., Carducci M.A., Chi K.N., et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. - DOI - PMC - PubMed
-
- Halabi S., Lin C.Y., Kelly W.K., Fizazi K.S., Moul J.W., Kaplan E.B., Morris M.J., Small E.J. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2014;32:671–677. doi: 10.1200/JCO.2013.52.3696. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

