Opportunities for Successful Stabilization of Poor Glass-Forming Drugs: A Stability-Based Comparison of Mesoporous Silica Versus Hot Melt Extrusion Technologies
- PMID: 31689980
- PMCID: PMC6920921
- DOI: 10.3390/pharmaceutics11110577
Opportunities for Successful Stabilization of Poor Glass-Forming Drugs: A Stability-Based Comparison of Mesoporous Silica Versus Hot Melt Extrusion Technologies
Abstract
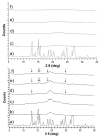
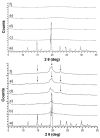
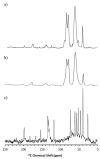
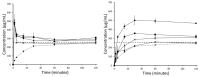
Amorphous formulation technologies to improve oral absorption of poorly soluble active pharmaceutical ingredients (APIs) have become increasingly prevalent. Currently, polymer-based amorphous formulations manufactured by spray drying, hot melt extrusion (HME), or co-precipitation are most common. However, these technologies have challenges in terms of the successful stabilization of poor glass former compounds in the amorphous form. An alternative approach is mesoporous silica, which stabilizes APIs in non-crystalline form via molecular adsorption inside nano-scale pores. In line with these considerations, two poor glass formers, haloperidol and carbamazepine, were formulated as polymer-based solid dispersion via HME and with mesoporous silica, and their stability was compared under accelerated conditions. Changes were monitored over three months with respect to solid-state form and dissolution. The results were supported by solid-state nuclear magnetic resonance spectroscopy (SS-NMR) and scanning electron microscopy (SEM). It was demonstrated that mesoporous silica was more successful than HME in the stabilization of the selected poor glass formers. While both drugs remained non-crystalline during the study using mesoporous silica, polymer-based HME formulations showed recrystallization after one week. Thus, mesoporous silica represents an attractive technology to extend the formulation toolbox to poorly soluble poor glass formers.
Keywords: amorphous stability; glass forming ability; hot melt extrusion; mesoporous silica; supersaturation.
Conflict of interest statement
Daniel J. Price, Anita Nair and Christoph Saal are full-time employees of Merck KGaA. Otherwise, the authors report no conflict of interest. The studies were performed under the auspices of the EU grant, including all funding, and independent of the company.
Figures





References
-
- Rams-Baron M., Jachowicz R., Boldyreva E., Zhou D., Jamroz W., Paluch M. Amorphous Drugs. Springer International Publishing; Cham, Switzerland: 2018. Physical Instability: A Key Problem of Amorphous Drugs; pp. 107–157.
Grants and funding
LinkOut - more resources
Full Text Sources

