Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent
- PMID: 31690018
- PMCID: PMC6864761
- DOI: 10.3390/molecules24213986
Single-Step Methylation of Chitosan Using Dimethyl Carbonate as a Green Methylating Agent
Abstract
N,N,N-Trimethyl chitosan (TMC) is one chitosan derivative that, because of its improved solubility, has been studied for industrial and pharmaceutic applications. Conventional methods for the synthesis of TMC involve the use of highly toxic and harmful reagents, such as methyl iodide and dimethyl sulfate (DMS). Although the methylation of dimethylated chitosan to TMC by dimethyl carbonate (DMC, a green and benign methylating agent) was reported recently, it involved a formaldehyde-based procedure. In this paper we report the single-step synthesis of TMC from chitosan using DMC in an ionic liquid. The TMC synthesised was characterised by 1H NMR spectroscopy and a functionally meaningful degree of quaternisation of 9% was demonstrated after a 12-h reaction time.
Keywords: N-methylation; dimethyl carbonate; green methylating agents; ionic liquids; trimethyl chitosan.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


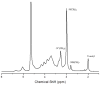
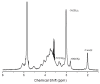
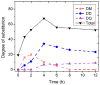
 ), dimethylation (DD,
), dimethylation (DD,  ) and quaternisation (DQ,
) and quaternisation (DQ,  ), and total degree of substitution (
), and total degree of substitution ( ) for TMC prepared using DMC as the methylating agent with varying reaction times (0–12 h).
) for TMC prepared using DMC as the methylating agent with varying reaction times (0–12 h).References
-
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. - DOI
-
- Mourya V.K., Inamdar N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008;68:1013–1051. doi: 10.1016/j.reactfunctpolym.2008.03.002. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

