Structure activity relationship studies on Amb639752: toward the identification of a common pharmacophoric structure for DGKα inhibitors
- PMID: 31690133
- PMCID: PMC6844378
- DOI: 10.1080/14756366.2019.1684911
Structure activity relationship studies on Amb639752: toward the identification of a common pharmacophoric structure for DGKα inhibitors
Abstract
A series of analogues of Amb639752, a novel diacylglycerol kinase (DGK) inhibitor recently discovered by us via virtual screening, have been tested. The compounds were evaluated as DGK inhibitors on α, θ, and ζ isoforms, and as antagonists on serotonin receptors. From these assays emerged two novel compounds, namely 11 and 20, which with an IC50 respectively of 1.6 and 1.8 µM are the most potent inhibitors of DGKα discovered to date. Both compounds demonstrated the ability to restore apoptosis in a cellular model of X-linked lymphoproliferative disease as well as the capacity to reduce the migration of cancer cells, suggesting their potential utility in preventing metastasis. Finally, relying on experimental biological data, molecular modelling studies allow us to set a three-point pharmacophore model for DGK inhibitors.
Keywords: Diacylglycerol kinase; enzyme assays; kinase inhibitors; molecular modelling; structure–activity relationship.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
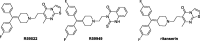









 PMA stimulated cells, ■ PMA and serotonin stimulated cells). Results are expressed as n moles of reduced cytochrome C/106 cells. Data are means ± SEM of 10 independent experiments performed in triplicate.
PMA stimulated cells, ■ PMA and serotonin stimulated cells). Results are expressed as n moles of reduced cytochrome C/106 cells. Data are means ± SEM of 10 independent experiments performed in triplicate.


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
