Paternal Grandmother Age Affects the Strength of Wolbachia-Induced Cytoplasmic Incompatibility in Drosophila melanogaster
- PMID: 31690673
- PMCID: PMC6831774
- DOI: 10.1128/mBio.01879-19
Paternal Grandmother Age Affects the Strength of Wolbachia-Induced Cytoplasmic Incompatibility in Drosophila melanogaster
Abstract
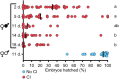
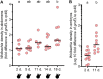
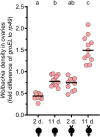
Wolbachia are obligate intracellular bacteria that are globally distributed in half of all arthropod species. As the most abundant maternally inherited microbe in animals, Wolbachia manipulate host reproduction via reproductive parasitism strategies, including cytoplasmic incompatibility (CI). CI manifests as embryonic death when Wolbachia-modified sperm fertilize uninfected eggs but not maternally infected eggs. Thus, CI can provide a relative fitness advantage to Wolbachia-infected females and drive the infection through a population. In the genetic model Drosophila melanogaster, the Wolbachia strain wMel induces variable CI, making mechanistic studies in D. melanogaster cumbersome. Here, we demonstrate that sons of older paternal D. melanogaster grandmothers induce stronger CI than sons of younger paternal grandmothers, and we term this relationship the "
Keywords: Drosophila melanogaster; Wolbachia; cytoplasmic incompatibility; maternal transmission.
Copyright © 2019 Layton et al.
Figures
References
-
- Ferri E, Bain O, Barbuto M, Martin C, Lo N, Uni S, Landmann F, Baccei SG, Guerrero R, de Souza Lima S, Bandi C, Wanji S, Diagne M, Casiraghi M. 2011. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS One 6:e20843. doi:10.1371/journal.pone.0020843. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

