Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation
- PMID: 31690717
- PMCID: PMC6831602
- DOI: 10.1038/s41467-019-12949-1
Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation
Abstract
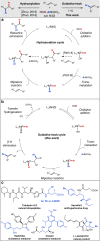
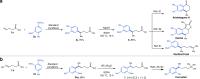
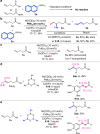
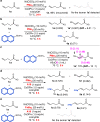
The conventional oxidative Heck reaction between aryl boronic acids and alkenes typically involved the PdII/Pd0/PdII catalytic cycle incorporating an external oxidant and often suffered C=C bond isomerization for internal alkyl-substituted alkenes via chain-walking. Herein, we demonstrate that the regioselectivity (γ-selectivity vs. δ-selectivity) and pathway selectivity (hydroarylation vs. oxidative Heck coupling) of a directed Ni-catalyzed alkene arylation can be controlled by judicious tuning of the coordination environment around the nickel catalyst via optimization of an appropriate phosphine ligand and directing group. In this way, the Ni(0)-catalyzed oxidative Heck arylation that relies on transfer hydrogenation of an acceptor olefin is developed with excellent E/Z selectivity and regioselectivity. Mechanistic investigations suggest that the addition of the acceptor is crucial for lowering the energy for carbometalation and for enabling catalytic turnover.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
From α-arylation of olefins to acylation with aldehydes: a journey in regiocontrol of the Heck reaction.Acc Chem Res. 2011 Aug 16;44(8):614-26. doi: 10.1021/ar200053d. Epub 2011 May 25. Acc Chem Res. 2011. PMID: 21612205 Review.
-
Mechanistic origin of ligand-controlled regioselectivity in Pd-catalyzed C-H activation/arylation of thiophenes.Chemistry. 2011 Dec 2;17(49):13866-76. doi: 10.1002/chem.201101587. Epub 2011 Nov 3. Chemistry. 2011. PMID: 22052569
-
Catalyst-Controlled Regioselectivity in Pd-Catalyzed Aerobic Oxidative Arylation of Indoles.Organometallics. 2021 Jul 26;40(14):2198-2203. doi: 10.1021/acs.organomet.1c00139. Epub 2021 Apr 8. Organometallics. 2021. PMID: 34366539 Free PMC article.
-
NiH-Catalyzed Functionalization of Remote and Proximal Olefins: New Reactions and Innovative Strategies.Acc Chem Res. 2022 Dec 6;55(23):3519-3536. doi: 10.1021/acs.accounts.2c00628. Epub 2022 Nov 9. Acc Chem Res. 2022. PMID: 36350093
-
Nickel and cobalt-catalyzed coupling of alkyl halides with alkenes via heck reactions and radical conjugate addition.Mini Rev Med Chem. 2013 May 1;13(6):802-13. doi: 10.2174/1389557511313060003. Mini Rev Med Chem. 2013. PMID: 23544460 Review.
Cited by
-
Directed Markovnikov hydroarylation and hydroalkenylation of alkenes under nickel catalysis.Chem Sci. 2021 Jul 13;12(33):11038-11044. doi: 10.1039/d1sc03121j. eCollection 2021 Aug 25. Chem Sci. 2021. PMID: 34522301 Free PMC article.
-
Ligand-enabled Ni-catalyzed hydroarylation and hydroalkenylation of internal alkenes with organoborons.Nat Commun. 2022 Nov 12;13(1):6878. doi: 10.1038/s41467-022-34675-x. Nat Commun. 2022. PMID: 36371437 Free PMC article.
-
Low-valent tungsten redox catalysis enables controlled isomerization and carbonylative functionalization of alkenes.Nat Chem. 2022 Jun;14(6):632-639. doi: 10.1038/s41557-022-00951-y. Epub 2022 Jun 2. Nat Chem. 2022. PMID: 35655006 Free PMC article.
-
γ-Selective C(sp3)-H amination via controlled migratory hydroamination.Nat Commun. 2021 Sep 27;12(1):5657. doi: 10.1038/s41467-021-25696-z. Nat Commun. 2021. PMID: 34580295 Free PMC article.
-
Divergent regioselective Heck-type reaction of unactivated alkenes and N-fluoro-sulfonamides.Nat Commun. 2022 Oct 22;13(1):6297. doi: 10.1038/s41467-022-33996-1. Nat Commun. 2022. PMID: 36272976 Free PMC article.
References
-
- Heck RF. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979;12:146–151. doi: 10.1021/ar50136a006. - DOI
-
- Oestreich, M. The Mizoroki–Heck Reaction (Wiley, Chichester, 2009).
Publication types
LinkOut - more resources
Full Text Sources

