Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation
- PMID: 31690717
- PMCID: PMC6831602
- DOI: 10.1038/s41467-019-12949-1
Nickel-catalyzed intermolecular oxidative Heck arylation driven by transfer hydrogenation
Abstract
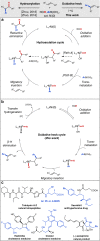
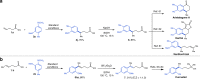
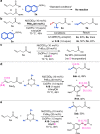
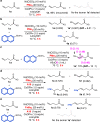
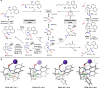
The conventional oxidative Heck reaction between aryl boronic acids and alkenes typically involved the PdII/Pd0/PdII catalytic cycle incorporating an external oxidant and often suffered C=C bond isomerization for internal alkyl-substituted alkenes via chain-walking. Herein, we demonstrate that the regioselectivity (γ-selectivity vs. δ-selectivity) and pathway selectivity (hydroarylation vs. oxidative Heck coupling) of a directed Ni-catalyzed alkene arylation can be controlled by judicious tuning of the coordination environment around the nickel catalyst via optimization of an appropriate phosphine ligand and directing group. In this way, the Ni(0)-catalyzed oxidative Heck arylation that relies on transfer hydrogenation of an acceptor olefin is developed with excellent E/Z selectivity and regioselectivity. Mechanistic investigations suggest that the addition of the acceptor is crucial for lowering the energy for carbometalation and for enabling catalytic turnover.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Heck RF. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979;12:146–151. doi: 10.1021/ar50136a006. - DOI
-
- Oestreich, M. The Mizoroki–Heck Reaction (Wiley, Chichester, 2009).
Publication types
LinkOut - more resources
Full Text Sources

