Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction
- PMID: 31690747
- PMCID: PMC6831672
- DOI: 10.1038/s41598-019-52336-w
Cannabinoids Exacerbate Alcohol Teratogenesis by a CB1-Hedgehog Interaction
Abstract
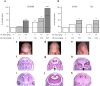
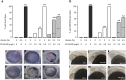
We tested whether cannabinoids (CBs) potentiate alcohol-induced birth defects in mice and zebrafish, and explored the underlying pathogenic mechanisms on Sonic Hedgehog (Shh) signaling. The CBs, Δ9-THC, cannabidiol, HU-210, and CP 55,940 caused alcohol-like effects on craniofacial and brain development, phenocopying Shh mutations. Combined exposure to even low doses of alcohol with THC, HU-210, or CP 55,940 caused a greater incidence of birth defects, particularly of the eyes, than did either treatment alone. Consistent with the hypothesis that these defects are caused by deficient Shh, we found that CBs reduced Shh signaling by inhibiting Smoothened (Smo), while Shh mRNA or a CB1 receptor antagonist attenuated CB-induced birth defects. Proximity ligation experiments identified novel CB1-Smo heteromers, suggesting allosteric CB1-Smo interactions. In addition to raising concerns about the safety of cannabinoid and alcohol exposure during early embryonic development, this study establishes a novel link between two distinct signaling pathways and has widespread implications for development, as well as diseases such as addiction and cancer.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Grant BF, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911–923. doi: 10.1001/jamapsychiatry.2017.2161. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

