Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation
- PMID: 31690792
- PMCID: PMC6831633
- DOI: 10.1038/s41598-019-52583-x
Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation
Abstract
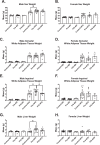
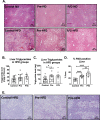
With the increasing prevalence of obesity in women of reproductive age there is a need to understand the ramifications of this on offspring. The purpose of this study is to investigate the programming effects of maternal obesity during preconception and the preconception/gestational period on adiposity and adipose tissue inflammation in offspring using an animal model. Adult female C57Bl/6J mice were assigned either normal diet, high fat diet (HFD) prior to pregnancy, or HFD prior to and through pregnancy. Some offspring were maintained on normal diet while others started HFD later in life. Offspring were assessed for body composition and metabolic responses. Lipid storing tissues were evaluated for expansion and inflammation. Male offspring from the preconception group had the greatest weight gain, most subcutaneous adipose tissue, and largest liver mass when introduced to postnatal HFD. Male offspring of the preconception/gestation group had worsened glucose tolerance and an increase in resident (CD11c-) adipose tissue macrophages (ATMs) when exposed to postnatal HFD. Female offspring had no significant difference in any parameter between the diet treatment groups. In conclusion, this study demonstrates that prenatal and pregnancy windows have independent programming effects on offspring. Preconception exposure affects body composition and adiposity while gestation exposure affects metabolism and tissue immune cell phenotypes.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

