Tumor angiogenesis: causes, consequences, challenges and opportunities
- PMID: 31690961
- PMCID: PMC7190605
- DOI: 10.1007/s00018-019-03351-7
Tumor angiogenesis: causes, consequences, challenges and opportunities
Abstract
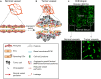
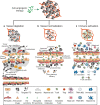
Tumor vascularization occurs through several distinct biological processes, which not only vary between tumor type and anatomic location, but also occur simultaneously within the same cancer tissue. These processes are orchestrated by a range of secreted factors and signaling pathways and can involve participation of non-endothelial cells, such as progenitors or cancer stem cells. Anti-angiogenic therapies using either antibodies or tyrosine kinase inhibitors have been approved to treat several types of cancer. However, the benefit of treatment has so far been modest, some patients not responding at all and others acquiring resistance. It is becoming increasingly clear that blocking tumors from accessing the circulation is not an easy task to accomplish. Tumor vessel functionality and gene expression often differ vastly when comparing different cancer subtypes, and vessel phenotype can be markedly heterogeneous within a single tumor. Here, we summarize the current understanding of cellular and molecular mechanisms involved in tumor angiogenesis and discuss challenges and opportunities associated with vascular targeting.
Keywords: Angiogenesis; Anti-angiogenic therapy; Cancer; Endothelial; VEGF; Vascular targeting.
Figures

References
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. - PubMed
-
- Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. - PubMed
-
- Kuczynski EA, et al. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16(8):469–493. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

