Isolation and characterization of phage AHP-1 and its combined effect with chloramphenicol to control Aeromonas hydrophila
- PMID: 31691176
- PMCID: PMC7058758
- DOI: 10.1007/s42770-019-00178-z
Isolation and characterization of phage AHP-1 and its combined effect with chloramphenicol to control Aeromonas hydrophila
Abstract
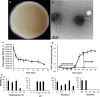
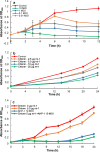
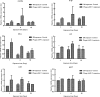
To develop an alternative bio-control measure for multi-drug resistant pathogenic Aeromonas hydrophila, which causes motile Aeromonas septicemia in fish, novel virulent phage (AHP-1) was isolated from carp tissues. Morphological analysis by transmission electron microscopy revealed that AHP-1 belongs to Myoviridae family. AHP-1 displayed 81% of moderate adsorption by 25 min, and latent period of 40 min with burst size of 97 PFU mL-1 at an optimal multiplicity of infection (MOI) 0.1. AHP-1 was stable over a broad range of pH (4-11), temperature (4-50 °C), and salinity (0.1-3.5%). Both time and MOI dependent in vitro A. hydrophila growth inhibition was observed with AHP-1. AHP-1 (10 MOI) showed higher growth inhibition against A. hydrophila than chloramphenicol (5 μg mL-1), and combined treatment was more promising than individuals. Immune gene expression analysis of zebrafish upon continuous bath exposure to AHP-1 resulted significantly higher (il-6 and sod-1) or slight induction (tnf-α, il1-β, il-10, and cxcl-8a) than controls at beginning of the phage exposure, but those lowered to basal level by day 12 post-phage exposure. It suggests no adverse immune responses have occurred for the AHP-1 dose that used, and have potential for the phage therapy. Further detailed in vivo studies are needed to confirm the protective efficacy of newly isolated AHP-1 against A. hydrophila infection.
Keywords: A. hydrophila; Chloramphenicol; Fish pathogen; Immune responses; Multi-drug resistance; Phage AHP-1.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

