AS1411 aptamer modified carbon dots via polyethylenimine-assisted strategy for efficient targeted cancer cell imaging
- PMID: 31691382
- PMCID: PMC6985679
- DOI: 10.1111/cpr.12713
AS1411 aptamer modified carbon dots via polyethylenimine-assisted strategy for efficient targeted cancer cell imaging
Abstract
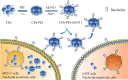
Objectives: Carbon dots (CDs), as a fascinating class of fluorescent carbon nanomaterials, have been proven to be powerful tools in the field of bioimaging and biosensing due to their small size, suitable photostability and favourable biocompatibility. However, the cellular uptake of free CDs lacks selectivity and the same negative charges as cell membranes may cause inefficient cell internalization. In this study, an efficient detecting and targeting nanosystem was developed based on the DNA aptamer AS1411 modified CDs with polyethyleneimine (PEI) as connecting bridge.
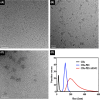
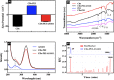
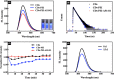
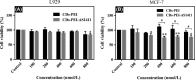
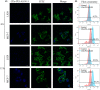
Materials and methods: Hydrothermally prepared CDs were assembled with positive-charged PEI, followed by conjugation with AS1411 through electrostatic interaction to form CDs-PEI-AS1411 nanocomplexes. The CDs, CDs-PEI and CDs-PEI-AS1411 were characterized by transmission electron microscopy (TEM), fourier transform infrared (FTIR) spectra, UV-vis spectra, zeta potential measurements and capillary electrophoresis characterizations. The cytotoxicity investigation of the CDs-PEI-AS1411 and CDs-PEI in both MCF-7 and L929 cells was carried out by the CCK-8 assay. The cellular uptake of the CDs-PEI-AS1411 was studied with confocal microscopy and flow cytometry.
Results: The as-prepared nanosystem possessed good photostability and no obvious cytotoxicity. On the basis of the confocal laser scanning microscope observation and the flow cytometry studies, the cellular uptake of CDs-PEI-AS1411 nanosystem in MCF-7 cells was significantly higher than that of L929 cells, which revealed the highly selective detection ability of nucleolin-positive cells.
Conclusions: The results of this study indicated that the CDs-PEI-AS1411 nanosystem had a potential value in cancer cell targeted imaging.
Keywords: AS1411 aptamer; carbon dots; polyethylenimine; targeted imaging.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
There are no conflicts of interests to declare.
Figures
References
-
- Zhang J, Zhao X, Xian M, et al. Folic acid‐conjugated green luminescent carbon dots as a nanoprobe for identifying folate receptor‐positive cancer cells. Talanta. 2018;183:39‐47. - PubMed
-
- Zhao Q, Wang S, Yang Y, et al. Hyaluronic acid and carbon dots‐gated hollow mesoporous silica for redox and enzyme‐triggered targeted drug delivery and bioimaging. Mater Sci Eng C Mater Biol Appl. 2017;78:475‐484. - PubMed
-
- Luo L, Liu C, He T, et al. Engineered fluorescent carbon dots as promising immune adjuvants to efficiently enhance cancer immunotherapy. Nanoscale. 2018;10:22035‐22043. - PubMed
-
- Li H, Guo L, Huang A, et al. Nanoparticle‐conjugated aptamer targeting hnRNP A2/B1 can recognize multiple tumor cells and inhibit their proliferation. Biomaterials. 2015;63:168‐176. - PubMed
-
- Lee CH, Rajendran R, Jeong MS, et al. Bioimaging of targeting cancers using aptamer‐conjugated carbon nanodots. Chem Commun (Camb). 2013;49:6543‐6545. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

