Ischemic stroke alters immune cell niche and chemokine profile in mice independent of spontaneous bacterial infection
- PMID: 31691533
- PMCID: PMC6842816
- DOI: 10.1002/iid3.277
Ischemic stroke alters immune cell niche and chemokine profile in mice independent of spontaneous bacterial infection
Abstract
Introduction: Stroke-associated pneumonia (SAP) is a major cause of mortality in patients who have suffered from severe ischemic stroke. Although multifactorial in nature, stroke-induced immunosuppression plays a key role in the development of SAP. Previous studies using a murine model of transient middle cerebral artery occlusion (tMCAO) have shown that focal ischemic stroke induction results in functional defects of lymphocytes in the spleen, thymus, and peripheral blood, leading to spontaneous bacterial infection in the lungs without inoculation. However, how ischemic stroke alters immune cell niche and the expression of cytokines and chemokines in the lungs has not been fully characterized.
Methods: Ischemic stroke was induced in mice by tMCAO. Immune cell profiles in the brain and the lungs at 24- and 72-hour time points were compared by flow cytometric analysis. Cytokine and chemokine expression in the lungs were determined by multiplex bead arrays. Tissue damage and bacterial burden in the lungs following tMCAO were evaluated.
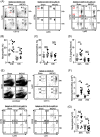
Results: Ischemic stroke increases the percentage of alveolar macrophages, neutrophils, and CD11b+ dendritic cells, but reduces the percentage of CD4+ T cells, CD8+ T cells, B cells, natural killer cells, and eosinophils in the lungs. The alteration of immune cell niche in the lungs coincides with a significant reduction in the levels of multiple chemokines in the lungs, including CCL3, CCL4, CCL5, CCL17, CCL20, CCL22, CXCL5, CXCL9, and CXCL10. Spontaneous bacterial infection and tissue damage following tMCAO, however, were not observed.
Conclusion: This is the first report to demonstrate a significant reduction of lymphocytes and multiple proinflammatory chemokines in the lungs following ischemic stroke in mice. These findings suggest that ischemic stroke directly impacts pulmonary immunity.
Keywords: chemokines; pulmonary immunity; stroke.
© 2019 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures








References
-
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654‐665. - PubMed
-
- Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37:2607‐2612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 GM109098/GM/NIGMS NIH HHS/United States
- U57GM104942/National Institute of General Medical Sciences, National Institutes of Health/International
- 5U54GM104942/National Institute of General Medical Sciences, National Institutes of Health/International
- P20GM109098/National Institute of General Medical Sciences, National Institutes of Health/International
- P20GM103434/National Institute of General Medical Sciences, National Institutes of Health/International
- U54 GM104942/GM/NIGMS NIH HHS/United States
- P20GM121322/National Institute of General Medical Sciences, National Institutes of Health/International
- P20 GM121322/GM/NIGMS NIH HHS/United States
- P20RR016440/National Institute of General Medical Sciences, National Institutes of Health/International
- S10OD016165/National Institute of General Medical Sciences, National Institutes of Health/International
- P30GM103488/National Institute of General Medical Sciences, National Institutes of Health/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

