Treatment of opioid withdrawal in neonates with morphine, phenobarbital, or chlorpromazine: a randomized double-blind trial
- PMID: 31691849
- PMCID: PMC6942588
- DOI: 10.1007/s00431-019-03486-6
Treatment of opioid withdrawal in neonates with morphine, phenobarbital, or chlorpromazine: a randomized double-blind trial
Abstract
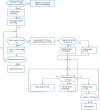
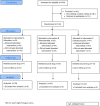
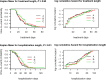
Three suitable compounds (morphine, chlorpromazine, and phenobarbital) to treat neonatal abstinence syndrome were compared in a prospective multicenter, double-blind trial. Neonates exposed to opioids in utero were randomly allocated to one of three treatment groups. When a predefined threshold of a modified Finnegan score was reached, treatment started and increased stepwise until symptoms were controlled. If symptoms could not be controlled with the predefined maximal dose of a single drug, a second drug was added. Among 143 infants recruited, 120 needed pharmacological treatment. Median length of treatment for morphine was 22 days (95% CI 18 to 33), for chlorpromazine 25 days (95% CI 21 to 34), and for phenobarbital 32 days (95% CI 27 to 38) (p = ns). In the morphine group, only 3% of infants (1/33) needed a second drug; in the chlorpromazine group, this proportion was 56% (24/43), and in the phenobarbital group 30% (13/44).Conclusion: None of the drugs tested for treating neonatal abstinence syndrome resulted in a significantly shorter treatment length than the others. As morphine alone was able to control symptoms in almost all infants, it may be preferred to the two other drugs but should still be tested against more potent opioids such as buprenorphine.Trial registration: At ClinicalTrials.gov NCT02810782 (registered retrospectively).What is Known:• Neonates exposed to opiates in utero and presenting with withdrawal symptoms should first be treated by non-pharmacological supportive measures.• In those who fail, drugs have to be given, but there is controversy which drug is best.What is New:• Among three candidates, morphine, chlorpromazine and phenobarbital, none resulted in significantly shorter treatment time.• As morphine alone was able to control symptoms in almost all infants, it may be preferred to the two other drugs.
Keywords: Chlorpromazine; Morphine; Neonate; Opioids; Pharmacological treatment; Phenobarbital; Withdrawal.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

