3-O-Sulfation of Heparan Sulfate Enhances Tau Interaction and Cellular Uptake
- PMID: 31692167
- PMCID: PMC6982596
- DOI: 10.1002/anie.201913029
3-O-Sulfation of Heparan Sulfate Enhances Tau Interaction and Cellular Uptake
Abstract
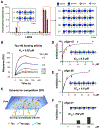
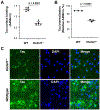
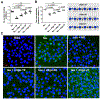
Prion-like transcellular spreading of tau in Alzheimer's Disease (AD) is mediated by tau binding to cell surface heparan sulfate (HS). However, the structural determinants for tau-HS interaction are not well understood. Microarray and SPR assays of structurally defined HS oligosaccharides show that a rare 3-O-sulfation (3-O-S) of HS significantly enhances tau binding. In Hs3st1-/- (HS 3-O-sulfotransferase-1 knockout) cells, reduced 3-O-S levels of HS diminished both cell surface binding and internalization of tau. In a cell culture, the addition of a 3-O-S HS 12-mer reduced both tau cell surface binding and cellular uptake. NMR titrations mapped 3-O-S binding sites to the microtubule binding repeat 2 (R2) and proline-rich region 2 (PRR2) of tau. Tau is only the seventh protein currently known to recognize HS 3-O-sulfation. Our work demonstrates that this rare 3-O-sulfation enhances tau-HS binding and likely the transcellular spread of tau, providing a novel target for disease-modifying treatment of AD and other tauopathies.
Keywords: Alzheimer's disease; cell surfaces; electrostatic interactions; heparan sulfate; proteins.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT, Neurology 1992, 42, 631–631. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

