Synthesis, crystal structures and electrochemical properties of ferrocenyl imidazole derivatives
- PMID: 31692585
- PMCID: PMC6806399
- DOI: 10.1016/j.heliyon.2019.e02580
Synthesis, crystal structures and electrochemical properties of ferrocenyl imidazole derivatives
Abstract
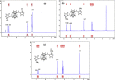
Six ferrocenyl imidazole derivatives substituted with -Cl, -NO2 and -CH3 on the 2-position of the 1H-imidazole ring have been synthesized. Of the six compounds, the di-substituted ferrocenes, i.e. compounds 4 (1,1'-ferrocenylmethyl(2-chloroimidazole)), 5 (1,1'-ferrocenyl(2-nitroimidazole)), and 6 (1,1'-ferrocenylmethyl(2-methylimidazole)) are reported for the first time. The structure-property relationships of compounds 4, 5 and 6 were investigated by means of UV-visible, FTIR, 1H-NMR, 13C-NMR spectroscopy and electrochemical studies. UV-visible analysis in acetonitrile showed that the π -π* band of compounds 2 (1-ferrocenylmethyl(2-nitroimidazole)) and 5 appeared at longer wavelength compared to 1 (1-ferrocenylmethyl(2-chloroimidazole)), 3 (1-ferrocenylmethyl(2-methylimidazole)), 4 and 6. This phenomenon is due to the different electronics around the imidazole moieties. In cyclic voltammetry analysis, all compounds exhibited a quasi-reversible redox wave for the ferrocenyl and imidazole moieties. Density functional theoretical (DFT) calculations with the B3LYP/6-311+G(d) basis set were performed on compounds 1-6, and the calculated HUMO-LUMO band gap energies correlated with those obtained from electrochemical and spectroscopic data. The X-ray crystallographic analysis highlighted the effect of electron-withdrawing and electron-donating substituents on the conformation of the cyclopentadienyl rings attached to the ferrocenyl moiety.
Keywords: Cyclic voltammetry; DFT calculations; Ferrocenyl imidazole derivatives; Materials chemistry; Theoretical chemistry; UV-vis spectroscopy.
© 2019 The Author(s).
Figures







References
-
- Yang W., Yin Z., Wang C.-H., Huang C., He J., Zhu X., Cheng J.-P. New redox anion receptors based on calix [4] pyrrole bearing ferrocene amide. Tetrahedron. 2008;64:9244–9252.
-
- Takase M., Inouye M. Ferrocene-modified bis (spiropyridopyran) s as synthetic signaling receptors for guanine–guanine dinucleoside derivatives. Chem. Commun. 2001;23:2432–2433. - PubMed
-
- Lorenzo Á., Aller E., Molina P. Iminophosphorane-based synthesis of multinuclear ferrocenyl urea, thiourea and guanidine derivatives and exploration of their anion sensing properties. Tetrahedron. 2009;65:1397–1401.
-
- Quintal S., Morais T.S., Matos C.P., Robalo M.P., Piedade M.F.M., de Brito M.J.V., Garcia M.H., Marques M., Maia C., Campino L. Synthesis, structural characterization and leishmanicidal activity evaluation of ferrocenyl N-heterocyclic compounds. J. Organomet. Chem. 2013;745:299–311.
-
- Niu H.-T., Yin Z., Su D., Niu D., Ao Y., He J., Cheng J.-P. Ferrocene-based imidazolium receptors for anions. Tetrahedron. 2008;64:6300–6306.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

