Mifepristone Followed by Misoprostol or Ethacridine Lactate and Oxytocin for Second Trimester Abortion: A Randomized Trial
- PMID: 31692613
- PMCID: PMC6812907
- DOI: 10.5152/eurasianjmed.2019.18341
Mifepristone Followed by Misoprostol or Ethacridine Lactate and Oxytocin for Second Trimester Abortion: A Randomized Trial
Abstract
Objective: To compare two medical methods for second-trimester abortion, mifepristone followed by misoprostol versus mifepristone followed by ethacridine lactate and oxytocin for success rate, induction to abortion time and acceptability.
Materials and methods: This is a randomized trial conducted from July 2014 to May 2016 and enrolled 120 women undergoing second trimester abortion (13-20 weeks). All patients received 200mg mifepristone orally and were randomized to receive further treatment after 36 hrs. Patients in Group M (n=60) received 400 microgram of misoprostol vaginally every 3 hours (maximum - 5 doses) and Group E (n=60) had extra-amniotic ethacridine lactate instillation followed by oxytocin infusion (max-100miu).
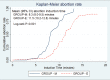
Results: Baseline demographic characteristics were comparable in both the groups. Success rate was 100% in group M and 98.3% in group E (p=0.31). Mean induction to abortion time was significantly shorter in group M than group E (8.2+2.3hours & 10.9+2.6 hours respectively; p=0.001). Majority of women reported side effects, 96.7% women in group M and 75% women in group E (p=0.001). Fall in hemoglobin after procedure was significantly higher in group M (0.70+0.33gram %) than group E (0.52+0.23 gram %) (p=0.001). Perception of intensity of pain was significantly more in group M but patient satisfaction in both groups was similar.
Conclusion: Both methods are comparable for success rate, induction interval was more for ethacridine lactate compared to misoprostol.
Keywords: Misoprostol; ethacridine lactate; second trimester abortion.
©Copyright 2019 by the Atatürk University School of Medicine - Available online at www.eurasianjmed.com.
Conflict of interest statement
Conflict of Interest: The authors declared no conflicts of interest.
Figures
Similar articles
-
Second trimester pregnancy termination including fetal death: comparison of five different methods.Eur J Obstet Gynecol Reprod Biol. 1996 Nov;69(2):97-102. doi: 10.1016/0301-2115(95)02548-0. Eur J Obstet Gynecol Reprod Biol. 1996. PMID: 8902440 Clinical Trial.
-
Randomized study on the effect of adding oxytocin to ethacridine lactate or misoprostol for second-trimester termination of pregnancy.Acta Obstet Gynecol Scand. 2006;85(7):825-9. doi: 10.1080/00016340500345337. Acta Obstet Gynecol Scand. 2006. PMID: 16817081 Clinical Trial.
-
Mifepristone followed by misoprostol or oxytocin for second-trimester abortion: a randomized controlled trial.Obstet Gynecol. 2013 Oct;122(4):815-820. doi: 10.1097/AOG.0b013e3182a2dcb7. Obstet Gynecol. 2013. PMID: 24084539 Clinical Trial.
-
Second trimester pregnancy termination using extra-amniotic ethacridine lactate.Br J Obstet Gynaecol. 1990 Nov;97(11):1026-9. doi: 10.1111/j.1471-0528.1990.tb02476.x. Br J Obstet Gynaecol. 1990. PMID: 2123711
-
Second-trimester postabortion care for ruptured membranes, fetal demise, and incomplete abortion.Int J Gynaecol Obstet. 2015 May;129(2):98-103. doi: 10.1016/j.ijgo.2014.11.011. Epub 2015 Jan 19. Int J Gynaecol Obstet. 2015. PMID: 25660084 Review.
Cited by
-
The Influence of Solvent on the Crystal Packing of Ethacridinium Phthalate Solvates.Materials (Basel). 2020 Nov 10;13(22):5073. doi: 10.3390/ma13225073. Materials (Basel). 2020. PMID: 33182832 Free PMC article.
References
-
- Safe abortion: technical and policy guidance for health systems. Second edition. World Health Organization; 2012. - PubMed
-
- Evidence based clinical guideline number 7. The care of Women requesting induced abortion. RCOG 2011.
LinkOut - more resources
Full Text Sources