Myeloid Tribbles 1 induces early atherosclerosis via enhanced foam cell expansion
- PMID: 31692955
- PMCID: PMC6821468
- DOI: 10.1126/sciadv.aax9183
Myeloid Tribbles 1 induces early atherosclerosis via enhanced foam cell expansion
Abstract
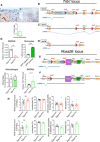
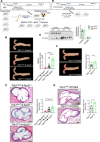
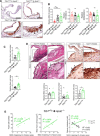
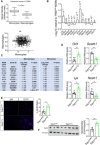
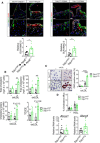
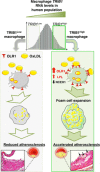
Macrophages drive atherosclerotic plaque progression and rupture; hence, attenuating their atherosclerosis-inducing properties holds promise for reducing coronary heart disease (CHD). Recent studies in mouse models have demonstrated that Tribbles 1 (Trib1) regulates macrophage phenotype and shows that Trib1 deficiency increases plasma cholesterol and triglyceride levels, suggesting that reduced TRIB1 expression mediates the strong genetic association between the TRIB1 locus and increased CHD risk in man. However, we report here that myeloid-specific Trib1 (mTrib1) deficiency reduces early atheroma formation and that mTrib1 transgene expression increases atherogenesis. Mechanistically, mTrib1 increased macrophage lipid accumulation and the expression of a critical receptor (OLR1), promoting oxidized low-density lipoprotein uptake and the formation of lipid-laden foam cells. As TRIB1 and OLR1 RNA levels were also strongly correlated in human macrophages, we suggest that a conserved, TRIB1-mediated mechanism drives foam cell formation in atherosclerotic plaque and that inhibiting mTRIB1 could be used therapeutically to reduce CHD.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures
References
-
- Rocha V. Z., Libby P., Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 6, 399–409 (2009). - PubMed
-
- Skålén K., Gustafsson M., Rydberg E. K., Hultén L. M., Wiklund O., Innerarity T. L., Borén J., Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417, 750–754 (2002). - PubMed
-
- Schrijvers D. M., De Meyer G. R., Kockx M. M., Herman A. G., Martinet W., Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 25, 1256–1261 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

