Potential Substitutes for Replacement of Lead in Perovskite Solar Cells: A Review
- PMID: 31692982
- PMCID: PMC6827533
- DOI: 10.1002/gch2.201900050
Potential Substitutes for Replacement of Lead in Perovskite Solar Cells: A Review
Abstract
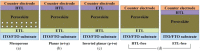
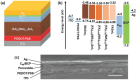
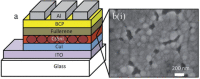
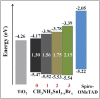
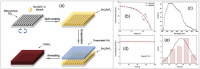
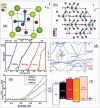
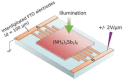
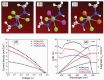
Lead halide perovskites have displayed the highest solar power conversion efficiencies of 23% but the toxicity issues of these materials need to be addressed. Lead-free perovskites have emerged as viable candidates for potential use as light harvesters to ensure clean and green photovoltaic technology. The substitution of lead by Sn, Ge, Bi, Sb, Cu and other potential candidates have reported efficiencies of up to 9%, but there is still a dire need to enhance their efficiencies and stability within the air. A comprehensive review is given on potential substitutes for lead-free perovskites and their characteristic features like energy bandgaps and optical absorption as well as photovoltaic parameters like open-circuit voltage (V OC), fill factor, short-circuit current density (J SC), and the device architecture for their efficient use. Lead-free perovskites do possess a suitable bandgap but have low efficiency. The use of additives has a significant effect on their efficiency and stability. The incorporation of cations like diethylammonium, phenylethyl ammonium, phenylethyl ammonium iodide, etc., or mixed cations at different compositions at the A-site is reported with engineered bandgaps having significant efficiency and stability. Recent work on the advancement of lead-free perovskites is also reviewed.
Keywords: lead‐free perovskites; photovoltaic parameters; stability.
© 2019 The Authors. Published by WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Tanaka H., Misono M., Curr. Opin. Solid State Mater. Sci. 2001, 5, 381.
-
- He T., Huang Q., Ramirez A., Wang Y., Regan K., Rogado N., Hayward M. A., Haas M. K., Slusky J. S., Inumara K., Zandbergen H. W., Ong N. P., Cava R. J., Nature 2001, 411, 54. - PubMed
-
- Goldschmidt V. M., Die Naturwiss. 1926, 14, 477.
-
- Liu X., Hong R., Tian C., J. Mater. Sci.: Mater. Electron. 2009, 20, 323.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

