Nuclear mechanosensing
- PMID: 31693005
- PMCID: PMC6830732
- DOI: 10.1042/ETLS20180051
Nuclear mechanosensing
Abstract
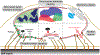
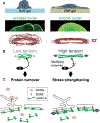
Structural links from the nucleus to the cytoskeleton and to the extracellular environment play a role in direct mechanosensing by nuclear factors. Here, we highlight recent studies that illustrate nuclear mechanosensation processes ranging from DNA repair and nuclear protein phospho-modulation to chromatin reorganization, lipase activation by dilation, and reversible rupture with the release of nuclear factors. Recent progresses demonstrate that these mechanosensing processes lead to modulation of gene expression such as those involved in the regulation of cytoskeletal programs and introduce copy number variations. The nuclear lamina protein lamin A has a recurring role, and various biophysical analyses prove helpful in clarifying mechanisms. The various recent observations provide further motivation to understand the regulation of nuclear mechanosensing pathways in both physiological and pathological contexts.
Conflict of interest statement
Competing Interests The Authors declare that there are no competing interests associated with the manuscript.
Figures
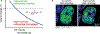

References
-
- Tamiello C, Kamps MA, van den Wijngaard A, Verstraeten VL, Baaijens FP, Broers JL et al. (2013) Soft substrates normalize nuclear morphology and prevent nuclear rupture in fibroblasts from a laminopathy patient with compound heterozygous LMNA mutations. Nucleus 4, 61–73 10.4161/nucl.23388 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
