Spectroscopic Evidence for Electronic Control of Heme Hydroxylation by IsdG
- PMID: 31693363
- PMCID: PMC7202882
- DOI: 10.1021/acs.inorgchem.9b02530
Spectroscopic Evidence for Electronic Control of Heme Hydroxylation by IsdG
Abstract
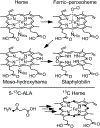
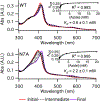
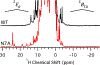
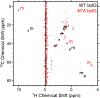
Staphylococcus aureus IsdG catalyzes a unique trioxygenation of heme to staphylobilin, and the data presented in this article elucidate the mechanism of the novel chemical transformation. More specifically, the roles of the second-sphere Asn and Trp residues in the monooxygenation of ferric-peroxoheme have been clarified via spectroscopic characterization of the ferric-azidoheme analogue. Analysis of UV/vis absorption data quantified the strength of the hydrogen bond that exists between the Asn7 side chain and the azide moiety of ferric-azidoheme. X-band electron paramagnetic resonance data were acquired and analyzed, which revealed that this hydrogen bond weakens the π-donor strength of the azide, resulting in perturbations of the Fe 3d based orbitals. Finally, nuclear magnetic resonance characterization of 13C-enriched samples demonstrated that the Asn7···N3 hydrogen bond triggers partial porphyrin to iron electron transfer, resulting in spin density delocalization onto the heme meso carbons. These spectroscopic experiments were complemented by combined quantum mechanics/molecular mechanics computational modeling, which strongly suggested that the electronic structure changes observed for the N7A variant arose from loss of the Asn7···N3 hydrogen bond as opposed to a decrease in porphyrin ruffling. From these data a fascinating picture emerges where an Asn7···N3 hydrogen bond is communicated through four bonds, resulting in meso carbons with partial cationic radical character that are poised for hydroxylation. This chemistry is not observed in other heme proteins because Asn7 and Trp67 must work in concert to trigger the requisite electronic structure change.
Figures





References
-
- Matsui T; Unno M; Ikeda-Saito M Heme oxygenase reveals its strategy for catalyzing three successive oxygenation reactions. Acc. Chem. Res. 2010, 43, 240–247. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

