Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma
- PMID: 31693429
- PMCID: PMC7032881
- DOI: 10.1200/JCO.19.00172
Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma
Abstract
Purpose: Patients with transplantation-ineligible relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) fare poorly, with limited treatment options. The antibody-drug conjugate polatuzumab vedotin targets CD79b, a B-cell receptor component.
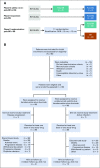
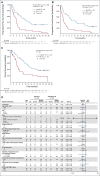
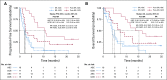
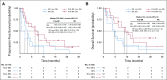
Methods: Safety and efficacy of polatuzumab vedotin with bendamustine and obinutuzumab (pola-BG) was evaluated in a single-arm cohort. Polatuzumab vedotin combined with bendamustine and rituximab (pola-BR) was compared with bendamustine and rituximab (BR) in a randomly assigned cohort of patients with transplantation-ineligible R/R DLBCL (primary end point: independent review committee [IRC] assessed complete response [CR] rate at the end of treatment). Duration of response, progression-free survival (PFS), and overall survival (OS) were analyzed using Kaplan-Meier and Cox regression methods.
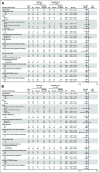
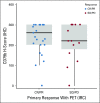
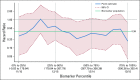
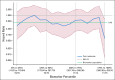
Results: Pola-BG and pola-BR had a tolerable safety profile. The phase Ib/II pola-BG cohort (n = 27) had a CR rate of 29.6% and a median OS of 10.8 months (median follow-up, 27.0 months). In the randomly assigned cohort (n = 80; 40 per arm), pola-BR patients had a significantly higher IRC-assessed CR rate (40.0% v 17.5%; P = .026) and longer IRC-assessed PFS (median, 9.5 v 3.7 months; hazard ratio [HR], 0.36, 95% CI, 0.21 to 0.63; P < .001) and OS (median, 12.4 v 4.7 months; HR, 0.42; 95% CI, 0.24 to 0.75; P = .002; median follow-up, 22.3 months). Pola-BR patients had higher rates of grade 3-4 neutropenia (46.2% v 33.3%), anemia (28.2% v 17.9%), and thrombocytopenia (41% v 23.1%), but similar grade 3-4 infections (23.1% v 20.5%), versus the BR group. Peripheral neuropathy associated with polatuzumab vedotin (43.6% of patients) was grade 1-2 and resolved in most patients.
Conclusion: Polatuzumab vedotin combined with BR resulted in a significantly higher CR rate and reduced the risk of death by 58% compared with BR in patients with transplantation-ineligible R/R DLBCL.
Trial registration: ClinicalTrials.gov NCT02257567.
Figures
Comment in
-
Polatuzumab Vedotin: Honing in on Relapsed or Refractory Diffuse Large B-Cell Lymphoma.J Clin Oncol. 2020 Jan 10;38(2):166-168. doi: 10.1200/JCO.19.02587. Epub 2019 Nov 26. J Clin Oncol. 2020. PMID: 31770050 No abstract available.
-
LyRIC indeterminate response and Immune-mediated pseudoprogression of diffuse large B-cell lymphoma following polatuzumab-based salvage therapy.Br J Haematol. 2020 Jun;189(6):e248-e251. doi: 10.1111/bjh.16679. Epub 2020 Apr 27. Br J Haematol. 2020. PMID: 32342503 No abstract available.
References
-
- Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. - PubMed
-
- Swerdlow SH, Campo E, Harris NL, et al: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Volume 2 (rev ed 4). Lyon, France, International Agency for Research on Cancer, 2017.
-
- Vitolo U, Trněný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35:3529–3537. - PubMed
-
- Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

