Aedes aegypti (Aag2)-derived clonal mosquito cell lines reveal the effects of pre-existing persistent infection with the insect-specific bunyavirus Phasi Charoen-like virus on arbovirus replication
- PMID: 31693659
- PMCID: PMC6860454
- DOI: 10.1371/journal.pntd.0007346
Aedes aegypti (Aag2)-derived clonal mosquito cell lines reveal the effects of pre-existing persistent infection with the insect-specific bunyavirus Phasi Charoen-like virus on arbovirus replication
Abstract
Background: Aedes aegypti is a vector mosquito of major public health importance, transmitting arthropod-borne viruses (arboviruses) such as chikungunya, dengue, yellow fever and Zika viruses. Wild mosquito populations are persistently infected at high prevalence with insect-specific viruses that do not replicate in vertebrate hosts. In experimental settings, acute infections with insect-specific viruses have been shown to modulate arbovirus infection and transmission in Ae. aegypti and other vector mosquitoes. However, the impact of persistent insect-specific virus infections, which arboviruses encounter more commonly in nature, has not been investigated extensively. Cell lines are useful models for studying virus-host interactions, however the available Ae. aegypti cell lines are poorly defined and heterogenous cultures.
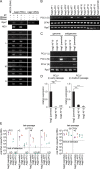
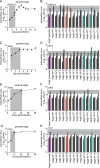
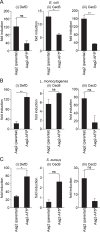
Methodology/principle findings: We generated single cell-derived clonal cell lines from the commonly used Ae. aegypti cell line Aag2. Two of the fourteen Aag2-derived clonal cell lines generated harboured markedly and consistently reduced levels of the insect-specific bunyavirus Phasi Charoen-like virus (PCLV) known to persistently infect Aag2 cells. In contrast to studies with acute insect-specific virus infections in cell culture and in vivo, we found that pre-existing persistent PCLV infection had no major impact on the replication of the flaviviruses dengue virus and Zika virus, the alphavirus Sindbis virus, or the rhabdovirus vesicular stomatitis virus. We also performed a detailed characterisation of the morphology, transfection efficiency and immune status of our Aag2-derived clonal cell lines, and have made a clone that we term Aag2-AF5 available to the research community as a well-defined cell culture model for arbovirus-vector interaction studies.
Conclusions/significance: Our findings highlight the need for further in vivo studies that more closely recapitulate natural arbovirus transmission settings in which arboviruses encounter mosquitoes harbouring persistent rather than acute insect-specific virus infections. Furthermore, we provide the well-characterised Aag2-derived clonal cell line as a valuable resource to the arbovirus research community.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

