Lactobacillus rhamnosus Lcr35 as an effective treatment for preventing Candida albicans infection in the invertebrate model Caenorhabditis elegans: First mechanistic insights
- PMID: 31693670
- PMCID: PMC6834333
- DOI: 10.1371/journal.pone.0216184
Lactobacillus rhamnosus Lcr35 as an effective treatment for preventing Candida albicans infection in the invertebrate model Caenorhabditis elegans: First mechanistic insights
Abstract
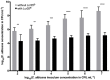
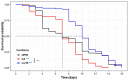
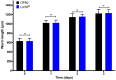
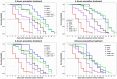
The increased recurrence of Candida albicans infections is associated with greater resistance to antifungal drugs. This involves the establishment of alternative therapeutic protocols, such as probiotic microorganisms whose antifungal potential has already been demonstrated using preclinical models (cell cultures, laboratory animals). Understanding the mechanisms of action of probiotic microorganisms has become a strategic need for the development of new therapeutics for humans. In this study, we investigated the prophylactic anti-C. albicans properties of Lactobacillus rhamnosus Lcr35® using the in vitro Caco-2 cell model and the in vivo Caenorhabditis elegans model. In Caco-2 cells, we showed that the strain Lcr35® significantly inhibited the growth (~2 log CFU.mL-1) and adhesion (150 to 6,300 times less) of the pathogen. Moreover, in addition to having a pro-longevity activity in the nematode (+42.9%, p = 3.56.10-6), Lcr35® protects the animal from the fungal infection (+267% of survival, p < 2.10-16) even if the yeast is still detectable in its intestine. At the mechanistic level, we noticed the repression of genes of the p38 MAPK signalling pathway and genes involved in the antifungal response induced by Lcr35®, suggesting that the pathogen no longer appears to be detected by the worm immune system. However, the DAF-16/FOXO transcription factor, implicated in the longevity and antipathogenic response of C. elegans, is activated by Lcr35®. These results suggest that the probiotic strain acts by stimulating its host via DAF-16 but also by suppressing the virulence of the pathogen.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: Adrien Nivoliez (AN) and Caroline Dausset (CD) had an institutional affiliation with the company biose, which manufactures Lcr35 products. The doctoral thesis of Cyril Poupet (CP) is partially financed by the company biose. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products to declare.
Figures
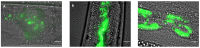
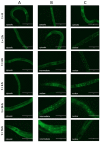
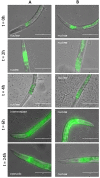
Similar articles
-
Curative Treatment of Candidiasis by the Live Biotherapeutic Microorganism Lactobacillus rhamnosus Lcr35® in the Invertebrate Model Caenorhabditis elegans: First Mechanistic Insights.Microorganisms. 2019 Dec 23;8(1):34. doi: 10.3390/microorganisms8010034. Microorganisms. 2019. PMID: 31878039 Free PMC article.
-
In vivo investigation of Lcr35® anti-candidiasis properties in Caenorhabditis elegans reveals the involvement of highly conserved immune pathways.Front Microbiol. 2022 Dec 23;13:1062113. doi: 10.3389/fmicb.2022.1062113. eCollection 2022. Front Microbiol. 2022. PMID: 36620055 Free PMC article.
-
Regulation of DAF-16-mediated longevity and immune response to Candida albicans infection in Caenorhabditis elegans.New Microbiol. 2022 Jan;45(1):51-61. Epub 2021 Dec 11. New Microbiol. 2022. PMID: 35403847
-
Action mechanisms of probiotics on Candida spp. and candidiasis prevention: an update.J Appl Microbiol. 2020 Aug;129(2):175-185. doi: 10.1111/jam.14511. Epub 2019 Nov 21. J Appl Microbiol. 2020. PMID: 31705713 Review.
-
DAF-16: FOXO in the Context of C. elegans.Curr Top Dev Biol. 2018;127:1-21. doi: 10.1016/bs.ctdb.2017.11.007. Epub 2018 Feb 2. Curr Top Dev Biol. 2018. PMID: 29433733 Review.
Cited by
-
Mucosal Bacteria Modulate Candida albicans Virulence in Oropharyngeal Candidiasis.mBio. 2021 Aug 31;12(4):e0193721. doi: 10.1128/mBio.01937-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399623 Free PMC article.
-
Curative Treatment of Candidiasis by the Live Biotherapeutic Microorganism Lactobacillus rhamnosus Lcr35® in the Invertebrate Model Caenorhabditis elegans: First Mechanistic Insights.Microorganisms. 2019 Dec 23;8(1):34. doi: 10.3390/microorganisms8010034. Microorganisms. 2019. PMID: 31878039 Free PMC article.
-
Lacticaseibacillus casei CNCM I-5663 supplementation maintained muscle mass in a model of frail rodents.Front Nutr. 2022 Aug 10;9:928798. doi: 10.3389/fnut.2022.928798. eCollection 2022. Front Nutr. 2022. PMID: 36034910 Free PMC article.
-
Evaluation of Probiotic Properties of Pediococcus acidilactici M76 Producing Functional Exopolysaccharides and Its Lactic Acid Fermentation of Black Raspberry Extract.Microorganisms. 2021 Jun 23;9(7):1364. doi: 10.3390/microorganisms9071364. Microorganisms. 2021. PMID: 34201704 Free PMC article.
-
A Mechanistic Study of the Antiaging Effect of Raw-Milk Cheese Extracts.Nutrients. 2021 Mar 10;13(3):897. doi: 10.3390/nu13030897. Nutrients. 2021. PMID: 33802038 Free PMC article.
References
-
- Cauchie M, Desmet S, Lagrou K. Candida and its dual lifestyle as a commensal and a pathogen. Res Microbiol [Internet]. 2017. November [cited 2018 Sep 5];168(9–10):802–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0923250817300402 - PubMed
-
- Neville BA, D’enfert C, Bougnoux M-E. Candida albicans commensalism in the gastrointestinal tract. FEMS Yeast Res [Internet]. 2015. [cited 2018 Sep 5];15:81 Available from: https://unite.ut.ee/ - PubMed
-
- Wächtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: Defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. Munro C, editor. PLoS One [Internet]. 2011. February 23 [cited 2018 Sep 10];6(2):e17046 Available from: http://www.ncbi.nlm.nih.gov/pubmed/21407800 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

