The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer
- PMID: 31693724
- PMCID: PMC6817454
- DOI: 10.18632/oncotarget.27239
The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer
Abstract
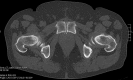
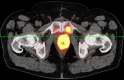
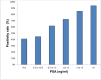
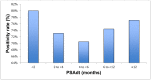
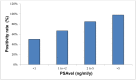
Background: The aim of the present study is to analyze the efficacy of 68Gallium (Ga)-PSMA-11 PET/CT for detecting and localizing recurrent prostate carcinoma (PC) in patients with different prostate-specific antigen (PSA), PSA velocity (PSAvel) and doubling time (PSAdt). Results: The PR of 68Ga-PSMA-11 PET/CT showed a positive relationship with PSA levels. Even at restaging PSA-values (PSAV) of lower than 0.2 ng/ml, PR was 41%. For PSAV of 0.2-<0.5 ng/ml the PR was 45%, 62% for PSAV of 0.5-<1.0 and 72% for PSAV of 1.0-<2.0 ng/ml. The PR increased to 85% for PSAV of 2.0-<5.0 and reached 94% at PSAV of ≥5.0 ng/ml. At PSA of <1 ng/ml/y the PR of PSAvel was 50% and increased to 98% at PSA >5 ng/ml/y. No significant association was found for PSAdt. Methods: PET/CT scans of 660 patients with biochemical recurrence (BCR) after primary therapy of PC were included in the analysis. We correlated serum PSA levels, measured at the time of imaging with PSMA PET/CT-positivity rates (PR) as well as PSAvel (in 225 patients) and PSAdt (660 patients). Additionally we compared the incidence of localized disease to metastases as related to these PSA-biomarkers. Conclusion: We have shown, in a large cohort of patients, that 68Ga-PSMA-11 PET/CT is a sensitive tool for restaging PC and has a high detection efficacy, even in patients with very low PSA levels (<0.2 ng/ml). Thus 68Ga-PSMA-11 PET/CT both identify and localize recurrent disease with implications for a more direct treatment approach (localized vs. systemic therapy).
Keywords: 68Gallium-PSMA PET/CT; biochemical recurrence; positivity rate; prostate cancer; prostate-specific antigen.
Copyright: © 2019 Hoffmann et al.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no conflict of interest. They have revealed to the Editors any relationships that they believe could be construed as resulting in an actual, potential, or perceived conflict of interest with regard to the manuscript submitted for review.
Figures
References
-
- Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, et al. , and PLCO Project Team . Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009; 360:1310–1319. 10.1056/NEJMoa0810696. - DOI - PMC - PubMed
-
- Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, van der Poel HG, van der Kwast TH, Rouvière O, et al. . EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017; 71:630–642. 10.1016/j.eururo.2016.08.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

