Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice
- PMID: 31694198
- PMCID: PMC6921031
- DOI: 10.3390/biom9110697
Chamaejasmine Isolated from Wikstroemia dolichantha Diels Suppresses 2,4-Dinitrofluoro-benzene-Induced Atopic Dermatitis in SKH-1 Hairless Mice
Abstract
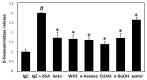
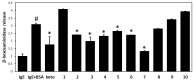
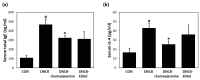
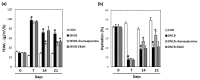
Plants of the genus Wikstroemia have long been used as traditional medicines to treat diseases like pneumonia, rheumatism, and bronchitis. This study was designed to determine the effect of chamaejasmine, a biflavonoid present in W. dolichantha, on atopic dermatitis (AD)-like skin lesions in a 2,4-dinitrochlorobenzene (DNCB)-induced murine model of AD. Initially, we examined the anti-allergic activities of ten flavonoids from W. dolichantha by measuring β-hexosaminidase release from RBL-2H3 cells. Subsequently, an SKH-1 hairless mouse model of AD was developed based on the topical application of DNCB. Chamaejasmine (0.5%) or pimecrolimus (1%, positive control) were applied to dorsal skins of DNCB-sensitized AD mice for two weeks. Serum IL-4 and IgE levels were determined using enzyme-linked immunosorbent assay kits and transepidermal water loss (TEWL) and skin hydration were measured using a Tewameter TM210 and a SKIN-O-MAT, respectively. Of the ten flavonoids isolated from W. dolichantha, chamaejasmine most potently inhibited DNP-specific IgE-induced degranulation in RBL-2H3 cells. Topical administration of chamaejasmine attenuated the clinical symptoms of DNCB-induced dermatitis (i.e., itching, dryness, erythema, and edema). Histological analyses demonstrated that dermal thickness and mast cell infiltration in dermis were significantly reduced by chamaejasmine. In addition, 0.5% chamaejasmine inhibited DNCB-induced increases in total IL-4 and IgE levels in serum, improved skin barrier function, and increased epidermis moisture. Our findings suggest chamaejasmine might be an effective therapeutic agent for the treatment of atopic diseases.
Keywords: 2,4-dinitrochlorobenzene; Wikstroemia dolichantha; atopic dermatitis; chamaejasmine; interleukin 4; skin barrier function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Boccardi D., D’Auria E., Turati F., DI M.V., Sortino S., Riva E., Cerri A. Disease severity and quality of life in children with atopic dermatitis: PO-SCORAD in clinical practice. Minerva Pediatr. 2017;69:373–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

