Carcinogenesis and Metastasis in Liver: Cell Physiological Basis
- PMID: 31694274
- PMCID: PMC6895858
- DOI: 10.3390/cancers11111731
Carcinogenesis and Metastasis in Liver: Cell Physiological Basis
Abstract
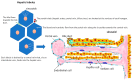
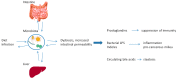
Hepatocellular carcinoma (HCC) incidence is rising. This paper summarises the current state of knowledge and recent discoveries in the cellular and physiological mechanisms leading to the development of liver cancer, especially HCC, and liver metastases. After reviewing normal hepatic cytoarchitecture and immunological characteristics, the paper addresses the pathophysiological factors that cause liver damage and predispose to neoplasia. Particular attention is given to chronic liver diseases, metabolic syndrome and the impact of altered gut microbiota, disrupted circadian rhythm and psychological stress. Improved knowledge of the multifactorial aetiology of HCC has important implications for the prevention and treatment of this cancer and of liver metastases in general.
Keywords: VEGF; circadian homeostasis; cortisol; hepatic stellate cells; hepatocellular carcinoma; immunity; myofibroblasts; nonalcoholic fatty liver disease; nonalcoholic steatohepatitis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Chang M.H., Chen C.J., Lai M.S., Hsu H.M., Wu T.C., Kong M.S., Liang D.C., Shau W.Y., Chen D.S. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. - DOI - PubMed
-
- Carrat F., Fontaine H., Dorival C., Simony M., Diallo A., Hezode C., De Ledinghen V., Larrey D., Haour G., Bronowicki J.-P., et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet. 2019;393:1453–1464. doi: 10.1016/S0140-6736(18)32111-1. - DOI - PubMed
-
- Schütte K., Schulz C., Poranzke J., Antweiler K., Bornschein J., Bretschneider T., Arend J., Ricke J., Malfertheiner P. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14:117. doi: 10.1186/1471-230X-14-117. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

