Enriching Personalized Endometrial Cancer Research with the Harmonization of Biobanking Standards
- PMID: 31694311
- PMCID: PMC6896027
- DOI: 10.3390/cancers11111734
Enriching Personalized Endometrial Cancer Research with the Harmonization of Biobanking Standards
Abstract
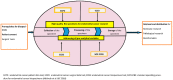
Endometrial cancer is the commonest gynecological cancer, with an incidence predicted to escalate by a further 50-100% before 2025, due to the rapid rise in risk factors such as obesity and increased life expectancy. Endometrial cancer associated mortality is also rising, depicting the need for translatable research to improve our understanding of the disease. Rapid translation of scientific discoveries will facilitate the development of new diagnostic, prognostic and therapeutic strategies. Biobanks play a vital role in providing biospecimens with accompanying clinical data for personalized translational research. Wide variation in collection, and pre-analytic variations in processing and storage of bio-specimens result in divergent and irreproducible data from multiple studies that are unsuitable for collation to formulate robust conclusions. Harmonization of biobanking standards is thus vital, in facilitating international multi-center collaborative studies with valuable outcomes to improve personalized treatments. This review will detail the pitfalls in the biobanking of biosamples from women with cancer in general, and describe the recent international harmonization project that developed standardized research tools to overcome these challenges and to enhance endometrial cancer research, which will facilitate future development of personalized novel diagnostic strategies and treatments.
Keywords: biobanking; biospecimens; endometrial cancer; harmonization; translational research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- CRUK. [(accessed on 29 September 2019)]; Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

