Halogenating Enzymes for Active Agent Synthesis: First Steps Are Done and Many Have to Follow
- PMID: 31694313
- PMCID: PMC6864650
- DOI: 10.3390/molecules24214008
Halogenating Enzymes for Active Agent Synthesis: First Steps Are Done and Many Have to Follow
Abstract
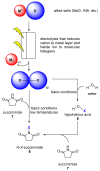
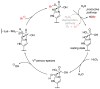
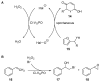
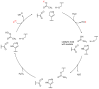
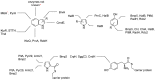
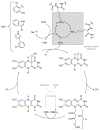
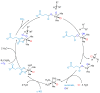
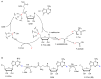
Halogens can be very important for active agents as vital parts of their binding mode, on the one hand, but are on the other hand instrumental in the synthesis of most active agents. However, the primary halogenating compound is molecular chlorine which has two major drawbacks, high energy consumption and hazardous handling. Nature bypassed molecular halogens and evolved at least six halogenating enzymes: Three kind of haloperoxidases, flavin-dependent halogenases as well as α-ketoglutarate and S-adenosylmethionine (SAM)-dependent halogenases. This review shows what is known today on these enzymes in terms of biocatalytic usage. The reader may understand this review as a plea for the usage of halogenating enzymes for fine chemical syntheses, but there are many steps to take until halogenating enzymes are reliable, flexible, and sustainable catalysts for halogenation.
Keywords: active agent synthesis; biocatalysis; bromination; chlorination; halogenase; haloperoxidase; pharmaceuticals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










Similar articles
-
Enzymatic chlorination and bromination.Methods Enzymol. 2012;516:237-57. doi: 10.1016/B978-0-12-394291-3.00004-6. Methods Enzymol. 2012. PMID: 23034232
-
Structural perspective on enzymatic halogenation.Acc Chem Res. 2009 Jan 20;42(1):147-55. doi: 10.1021/ar800088r. Acc Chem Res. 2009. PMID: 18774824 Free PMC article. Review.
-
Flavin-dependent halogenases involved in secondary metabolism in bacteria.Appl Microbiol Biotechnol. 2006 May;70(6):631-41. doi: 10.1007/s00253-005-0232-2. Epub 2006 Mar 17. Appl Microbiol Biotechnol. 2006. PMID: 16544142 Review.
-
Enzymatic Halogenases and Haloperoxidases: Computational Studies on Mechanism and Function.Adv Protein Chem Struct Biol. 2015;100:113-51. doi: 10.1016/bs.apcsb.2015.06.001. Epub 2015 Jul 8. Adv Protein Chem Struct Biol. 2015. PMID: 26415843 Review.
-
Catalytic mechanisms, basic roles, and biotechnological and environmental significance of halogenating enzymes.Acta Biochim Biophys Sin (Shanghai). 2008 Mar;40(3):183-93. doi: 10.1111/j.1745-7270.2008.00390.x. Acta Biochim Biophys Sin (Shanghai). 2008. PMID: 18330472 Review.
Cited by
-
Biosynthesis of natural and halogenated plant monoterpene indole alkaloids in yeast.Nat Chem Biol. 2023 Dec;19(12):1551-1560. doi: 10.1038/s41589-023-01430-2. Epub 2023 Nov 6. Nat Chem Biol. 2023. PMID: 37932529 Free PMC article.
-
Engineering Saccharomyces cerevisiae to produce plant benzylisoquinoline alkaloids.aBIOTECH. 2021;2(3):264-275. doi: 10.1007/s42994-021-00055-0. Epub 2021 Jul 18. aBIOTECH. 2021. PMID: 34377581 Free PMC article. Review.
-
Enzymatic strategies for asymmetric synthesis.RSC Chem Biol. 2021 Jun 1;2(4):958-989. doi: 10.1039/d1cb00080b. eCollection 2021 Aug 5. RSC Chem Biol. 2021. PMID: 34458820 Free PMC article. Review.
-
Nothing lasts forever: understanding microbial biodegradation of polyfluorinated compounds and perfluorinated alkyl substances.Microb Biotechnol. 2022 Mar;15(3):773-792. doi: 10.1111/1751-7915.13928. Epub 2021 Sep 27. Microb Biotechnol. 2022. PMID: 34570953 Free PMC article. Review.
-
Flavin Adenine Dinucleotide-Dependent Halogenase XanH and Engineering of Multifunctional Fusion Halogenases.Appl Environ Microbiol. 2020 Sep 1;86(18):e01225-20. doi: 10.1128/AEM.01225-20. Print 2020 Sep 1. Appl Environ Microbiol. 2020. PMID: 32651204 Free PMC article.
References
-
- Gribble G.W. Natural Organohalogens: A New Frontier for Medicinal Agents? J. Chem. Educ. 2004;81:1441. doi: 10.1021/ed081p1441. - DOI
-
- Chast F. The Practice of Medicinal Chemistry. Elsevier; Amsterdam, The Netherlands: 2008. A history of drug discovery: From first steps of chemistry to achievements in molecular pharmacology; pp. 1–62.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

