Comparison of CBF Measured with Combined Velocity-Selective Arterial Spin-Labeling and Pulsed Arterial Spin-Labeling to Blood Flow Patterns Assessed by Conventional Angiography in Pediatric Moyamoya
- PMID: 31694821
- PMCID: PMC6975103
- DOI: 10.3174/ajnr.A6262
Comparison of CBF Measured with Combined Velocity-Selective Arterial Spin-Labeling and Pulsed Arterial Spin-Labeling to Blood Flow Patterns Assessed by Conventional Angiography in Pediatric Moyamoya
Abstract
Background and purpose: Imaging CBF is important for managing pediatric moyamoya. Traditional arterial spin-labeling MR imaging detects delayed transit thorough diseased arteries but is inaccurate for measuring perfusion because of these delays. Velocity-selective arterial spin-labeling is insensitive to transit delay and well-suited for imaging Moyamoya perfusion. This study assesses the accuracy of a combined velocity-selective arterial spin-labeling and traditional pulsed arterial spin-labeling CBF approach in pediatric moyamoya, with comparison to blood flow patterns on conventional angiography.
Materials and methods: Twenty-two neurologically stable pediatric patients with moyamoya and 5 asymptomatic siblings without frank moyamoya were imaged with velocity-selective arterial spin-labeling, pulsed arterial spin-labeling, and DSA (patients). Qualitative comparison was performed, followed by a systematic comparison using ASPECTS-based scoring. Quantitative pulsed arterial spin-labeling CBF and velocity-selective arterial spin-labeling CBF for the middle cerebral artery, anterior cerebral artery, and posterior cerebral artery territories were also compared.
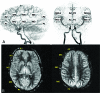
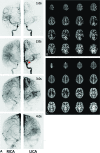
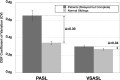
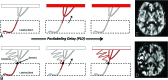
Results: Qualitatively, velocity-selective arterial spin-labeling perfusion maps reflect the DSA parenchymal phase, regardless of postinjection timing. Conversely, pulsed arterial spin-labeling maps reflect the DSA appearance at postinjection times closer to the arterial spin-labeling postlabeling delay, regardless of vascular phase. ASPECTS comparison showed excellent agreement (88%, κ = 0.77, P < .001) between arterial spin-labeling and DSA, suggesting velocity-selective arterial spin-labeling and pulsed arterial spin-labeling capture key perfusion and transit delay information, respectively. CBF coefficient of variation, a marker of perfusion variability, was similar for velocity-selective arterial spin-labeling in patient regions of delayed-but-preserved perfusion compared to healthy asymptomatic sibling regions (coefficient of variation = 0.30 versus 0.26, respectively, Δcoefficient of variation = 0.04), but it was significantly different for pulsed arterial spin-labeling (coefficient of variation = 0.64 versus 0.34, Δcoefficient of variation = 0.30, P < .001).
Conclusions: Velocity-selective arterial spin-labeling offers a powerful approach to image perfusion in pediatric moyamoya due to transit delay insensitivity. Coupled with pulsed arterial spin-labeling for transit delay information, a volumetric MR imaging approach capturing key DSA information is introduced.
© 2019 by American Journal of Neuroradiology.
Figures
Similar articles
-
Quantitative cerebral perfusion imaging in children and young adults with Moyamoya disease: comparison of arterial spin-labeling-MRI and H(2)[(15)O]-PET.AJNR Am J Neuroradiol. 2014 May;35(5):1022-8. doi: 10.3174/ajnr.A3799. Epub 2013 Dec 12. AJNR Am J Neuroradiol. 2014. PMID: 24335546 Free PMC article.
-
Arterial spin-labeling cerebral perfusion changes after revascularization surgery in pediatric moyamoya disease and syndrome.J Neurosurg Pediatr. 2019 Apr 1;23(4):486-492. doi: 10.3171/2018.11.PEDS18498. Epub 2019 Feb 8. J Neurosurg Pediatr. 2019. PMID: 30738390
-
Noninvasive Evaluation of CBF and Perfusion Delay of Moyamoya Disease Using Arterial Spin-Labeling MRI with Multiple Postlabeling Delays: Comparison with 15O-Gas PET and DSC-MRI.AJNR Am J Neuroradiol. 2017 Apr;38(4):696-702. doi: 10.3174/ajnr.A5068. Epub 2017 Feb 16. AJNR Am J Neuroradiol. 2017. PMID: 28209582 Free PMC article.
-
Clinical utility of arterial spin labeling imaging in disorders of the nervous system.Neurosurg Focus. 2019 Dec 1;47(6):E5. doi: 10.3171/2019.9.FOCUS19567. Neurosurg Focus. 2019. PMID: 31786550 Review.
-
Contemporary and emerging magnetic resonance imaging methods for evaluation of moyamoya disease.Neurosurg Focus. 2019 Dec 1;47(6):E6. doi: 10.3171/2019.9.FOCUS19616. Neurosurg Focus. 2019. PMID: 31786551 Review.
Cited by
-
Velocity-selective excitation: Principles and applications.NMR Biomed. 2023 Feb;36(2):e4820. doi: 10.1002/nbm.4820. Epub 2022 Sep 9. NMR Biomed. 2023. PMID: 35994473 Free PMC article. Review.
-
Alteration of intracranial blood perfusion in temporal lobe epilepsy, an arterial spin labeling study.Heliyon. 2023 Mar 24;9(4):e14854. doi: 10.1016/j.heliyon.2023.e14854. eCollection 2023 Apr. Heliyon. 2023. PMID: 37089370 Free PMC article.
-
Spatial dependency and the role of local susceptibility for velocity selective arterial spin labeling (VS-ASL) relative tagging efficiency using accelerated 3D radial sampling with a BIR-8 preparation.Magn Reson Med. 2021 Jul;86(1):293-307. doi: 10.1002/mrm.28726. Epub 2021 Feb 21. Magn Reson Med. 2021. PMID: 33615527 Free PMC article.
-
Advanced pediatric neuroimaging.Pediatr Radiol. 2023 Jun;53(7):1314-1323. doi: 10.1007/s00247-022-05519-z. Epub 2022 Oct 11. Pediatr Radiol. 2023. PMID: 36216985 Review.
-
Velocity-Selective Arterial Spin Labeling Perfusion in Monitoring High Grade Gliomas Following Therapy: Clinical Feasibility at 1.5T and Comparison with Dynamic Susceptibility Contrast Perfusion.Brain Sci. 2024 Jan 25;14(2):126. doi: 10.3390/brainsci14020126. Brain Sci. 2024. PMID: 38391701 Free PMC article.
References
-
- Wong EC, Cronin M, Wu WC, et al. . Velocity-selective arterial spin labeling. Magn Reson Med 2006;55:1334–41 - PubMed
