Methods for the early detection of drug-induced pancreatitis: a systematic review of the literature
- PMID: 31694842
- PMCID: PMC6858245
- DOI: 10.1136/bmjopen-2018-027451
Methods for the early detection of drug-induced pancreatitis: a systematic review of the literature
Abstract
Objectives: We systematically reviewed the literature to identify evidence-informed recommendations regarding the detection of drug-induced pancreatitis (DIP) and, secondarily, to describe clinical processes for the diagnosis of DIP.
Design: Systematic review.
Data sources: Ovid MEDLINE, including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Embase Classic+Embase, the Cochrane Library.
Eligibility criteria: We included clinical practice guidelines, systematic reviews, narrative reviews and observational studies with a focus of establishing incidence, prevalence or diagnostic approaches for DIP. Clinical trials that diagnosed DIP as an outcome were also included.
Data extraction and synthesis: Two reviewers screened citations and performed data extraction. A narrative synthesis of the evidence was prepared.
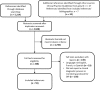
Results: Fifty-nine studies were included. Early published evidence suggested serial pancreatic ultrasound could detect subclinical pancreatitis; however, subsequent studies demonstrated no utility of serial ultrasound or serial monitoring of pancreatic enzymes in the early detection of DIP. Two small studies conducted in patients with a high baseline risk of acute pancreatitis concluded serial monitoring of pancreatic enzymes may be useful to guide early discontinuation of medications with known associations with pancreatitis. Early discontinuation of medication was not advised for lower-risk patients because some medications cause transient elevations of pancreatic enzymes that do not progress to acute pancreatitis. Eight of 52 studies (15%) reporting a clinical diagnostic process for DIP reported using currently accepted criteria for the diagnosis of acute pancreatitis. A variety of methods were used to assess drug-related causality.
Conclusions: There is minimal evidence to support the use of serial monitoring by ultrasound or pancreatic enzymes to detect cases of DIP. Serial monitoring may be useful to guide early discontinuation of DIP-associated drugs in high-risk patients, but not in lower-risk patients. Greater uptake of standardised diagnostic and causality criteria for DIP is needed.
Trial registration number: CRD42017060473.
Keywords: detection; diagnosis; drug reaction; pancreatitis; systematic review.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BH has previously received honoraria from Cornerstone Research Group for methodologic advice related to the conduct of systematic reviews and meta-analysis.
Figures
Similar articles
-
Drug induced pancreatitis: A systematic review of case reports to determine potential drug associations.PLoS One. 2020 Apr 17;15(4):e0231883. doi: 10.1371/journal.pone.0231883. eCollection 2020. PLoS One. 2020. PMID: 32302358 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Clinical utility of serologic testing for celiac disease in ontario: an evidence-based analysis.Ont Health Technol Assess Ser. 2010;10(21):1-111. Epub 2010 Dec 1. Ont Health Technol Assess Ser. 2010. PMID: 23074399 Free PMC article.
-
Frequency and prognosis of acute pancreatitis associated with fulminant or non-fulminant acute hepatitis A: A systematic review.Pancreatology. 2017 Mar-Apr;17(2):166-175. doi: 10.1016/j.pan.2017.02.008. Epub 2017 Feb 17. Pancreatology. 2017. PMID: 28236520
Cited by
-
Acute Pancreatitis: Diagnosis and Treatment.Drugs. 2022 Aug;82(12):1251-1276. doi: 10.1007/s40265-022-01766-4. Epub 2022 Sep 8. Drugs. 2022. PMID: 36074322 Free PMC article. Review.
-
A Case of Mirtazapine-Induced Pancreatitis.Cureus. 2023 Apr 4;15(4):e37129. doi: 10.7759/cureus.37129. eCollection 2023 Apr. Cureus. 2023. PMID: 37153315 Free PMC article.
-
Ever-increasing diversity of drug-induced pancreatitis.World J Gastroenterol. 2020 Jun 14;26(22):2902-2915. doi: 10.3748/wjg.v26.i22.2902. World J Gastroenterol. 2020. PMID: 32587438 Free PMC article. Review.
References
-
- Unalp-Arida A, Ruhl CE. The burden of acute pancreatitis in the United States population. Gastroenterology 2017;152:S289 10.1016/S0016-5085(17)31255-6 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical