The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia
- PMID: 31694851
- PMCID: PMC6858099
- DOI: 10.1136/bmjopen-2019-032122
The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia
Abstract
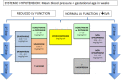
Introduction: Congenital diaphragmatic hernia (CDH) is a developmental defect of the diaphragm that impairs normal lung development, causing pulmonary hypertension (PH). PH in CDH newborns is the main determinant for morbidity and mortality. Different therapies are still mainly based on 'trial and error'. Inhaled nitric oxide (iNO) is often the drug of first choice. However, iNO does not seem to improve mortality. Intravenous sildenafil has reduced mortality in newborns with PH without CDH, but prospective data in CDH patients are lacking.
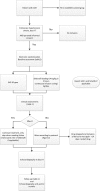
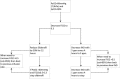
Methods and analysis: In an open label, multicentre, international randomised controlled trial in Europe, Canada and Australia, 330 newborns with CDH and PH are recruited over a 4-year period (2018-2022). Patients are randomised for intravenous sildenafil or iNO. Sildenafil is given in a loading dose of 0.4 mg/kg in 3 hours; followed by continuous infusion of 1.6 mg/kg/day, iNO is dosed at 20 ppm. Primary outcome is absence of PH on day 14 without pulmonary vasodilator therapy and/or absence of death within the first 28 days of life. Secondary outcome measures include clinical and echocardiographic markers of PH in the first year of life. We hypothesise that sildenafil gives a 25% reduction in the primary outcome from 68% to 48% on day 14, for which a sample size of 330 patients is needed. An intention-to-treat analysis will be performed. A p-value (two-sided) <0.05 is considered significant in all analyses.
Ethics and dissemination: Ethics approval has been granted by the ethics committee in Rotterdam (MEC-2017-324) and the central Committee on Research Involving Human Subjects (NL60229.078.17) in the Netherlands. The principles of the Declaration of Helsinki, the Medical Research Involving Human Subjects Act and the national rules and regulations on personal data protection will be used. Parental informed consent will be obtained.
Trial registration number: NTR6982; Pre-results.
Keywords: congenital diaphragmatic hernia; nitric oxide; pulmonary hypertension; sildenafil; therapeutics.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Galiè N, Hoeper MM, Humbert M, et al. . Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of cardiology (ESC) and the European respiratory Society (ERS), endorsed by the International Society of heart and lung transplantation (ISHLT). Eur Heart J 2009;30:2493–537. 10.1093/eurheartj/ehp297 - DOI - PubMed
-
- Russo FM, Eastwood MP, Keijzer R, et al. . Lung size and liver herniation predict need for extracorporeal membrane oxygenation but not pulmonary hypertension in isolated congenital diaphragmatic hernia: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;49:704–13. 10.1002/uog.16000 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
