Activation of NLRX1 by NX-13 Alleviates Inflammatory Bowel Disease through Immunometabolic Mechanisms in CD4+ T Cells
- PMID: 31694910
- PMCID: PMC6904519
- DOI: 10.4049/jimmunol.1900364
Activation of NLRX1 by NX-13 Alleviates Inflammatory Bowel Disease through Immunometabolic Mechanisms in CD4+ T Cells
Abstract
Inflammatory bowel disease (IBD) is a complex autoimmune disease with dysfunction in pattern-recognition responses, including within the NLR family. Nucleotide-binding oligomerization domain, leucine rich repeat containing X1 (NLRX1) is a unique NLR with regulatory and anti-inflammatory functions resulting in protection from IBD in mouse models. NX-13 is an orally active, gut-restricted novel drug candidate that selectively targets and activates the NLRX1 pathway locally in the gut. In vitro and in vivo efficacy of NLRX1 activation by NX-13 was examined. Oral treatment with NX-13 alleviates disease severity, colonic leukocytic infiltration, and cytokine markers of inflammation in three mouse models of IBD (dextran sulfate sodium, Mdr1a-/-, and CD45RBhi adoptive transfer). Treatment of naive CD4+ T cells with NX-13 in vitro decreases differentiation into Th1 and Th17 subsets with increased oxidative phosphorylation and decreased NF-κB activation and reactive oxygen species. With stimulation by PMA/ionomycin, TNF-α, or H2O2, PBMCs from ulcerative colitis patients treated with NX-13 had decreased NF-κB activity, TNF-α+ and IFN-γ+ CD4+ T cells and overall production of IL-6, MCP1, and IL-8. NX-13 activates NLRX1 to mediate a resistance to both inflammatory signaling and oxidative stress in mouse models and human primary cells from ulcerative colitis patients with effects on NF-κB activity and oxidative phosphorylation. NX-13 is a promising oral, gut-restricted NLRX1 agonist for treating IBD.
Copyright © 2019 by The American Association of Immunologists, Inc.
Figures


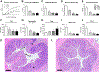



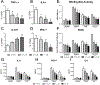
Similar articles
-
NLRX1 Regulates Effector and Metabolic Functions of CD4+ T Cells.J Immunol. 2017 Mar 15;198(6):2260-2268. doi: 10.4049/jimmunol.1601547. Epub 2017 Feb 3. J Immunol. 2017. PMID: 28159898 Free PMC article.
-
Exploratory studies with NX-13: oral toxicity and pharmacokinetics in rodents of an orally active, gut-restricted first-in-class therapeutic for IBD that targets NLRX1.Drug Chem Toxicol. 2022 Jan;45(1):209-214. doi: 10.1080/01480545.2019.1679828. Epub 2019 Oct 25. Drug Chem Toxicol. 2022. PMID: 31650868 Free PMC article.
-
NLRX1 Modulates Immunometabolic Mechanisms Controlling the Host-Gut Microbiota Interactions during Inflammatory Bowel Disease.Front Immunol. 2018 Feb 26;9:363. doi: 10.3389/fimmu.2018.00363. eCollection 2018. Front Immunol. 2018. PMID: 29535731 Free PMC article.
-
Negative regulatory NLRs mitigate inflammation via NF-κB pathway signaling in inflammatory bowel disease.Biomed J. 2023 Oct;46(5):100616. doi: 10.1016/j.bj.2023.100616. Epub 2023 Jun 14. Biomed J. 2023. PMID: 37321320 Free PMC article. Review.
-
NLRX1 Is a Multifaceted and Enigmatic Regulator of Immune System Function.Front Immunol. 2019 Oct 11;10:2419. doi: 10.3389/fimmu.2019.02419. eCollection 2019. Front Immunol. 2019. PMID: 31681307 Free PMC article. Review.
Cited by
-
The interaction of O-GlcNAc-modified NLRX1 and IKK-α modulates IL-1β expression in M1 macrophages.In Vitro Cell Dev Biol Anim. 2022 May;58(5):408-418. doi: 10.1007/s11626-022-00654-1. Epub 2022 May 5. In Vitro Cell Dev Biol Anim. 2022. PMID: 35513753
-
The regulation of self-tolerance and the role of inflammasome molecules.Front Immunol. 2023 Apr 4;14:1154552. doi: 10.3389/fimmu.2023.1154552. eCollection 2023. Front Immunol. 2023. PMID: 37081890 Free PMC article. Review.
-
Trust Your Gut: Strategies and Tactics for Intestinally Restricted Drugs.ACS Med Chem Lett. 2023 Feb 22;14(3):233-243. doi: 10.1021/acsmedchemlett.3c00001. eCollection 2023 Mar 9. ACS Med Chem Lett. 2023. PMID: 36923921 Free PMC article. Review.
-
NLRX1 Deletion Increases Ischemia-Reperfusion Damage and Activates Glucose Metabolism in Mouse Heart.Front Immunol. 2020 Dec 11;11:591815. doi: 10.3389/fimmu.2020.591815. eCollection 2020. Front Immunol. 2020. PMID: 33362773 Free PMC article.
-
The NLR gene family: from discovery to present day.Nat Rev Immunol. 2023 Oct;23(10):635-654. doi: 10.1038/s41577-023-00849-x. Epub 2023 Mar 27. Nat Rev Immunol. 2023. PMID: 36973360 Free PMC article. Review.
References
-
- Baumgart DC, and Sandborn WJ. 2012. Crohn’s disease. Lancet 380: 1590–1605. - PubMed
-
- Torres J, Mehandru S, Colombel JF, and Peyrin-Biroulet L. 2017. Crohn’s disease. Lancet 389: 1741–1755. - PubMed
-
- Ordas I, Eckmann L, Talamini M, Baumgart DC, and Sandborn WJ. 2012. Ulcerative colitis. Lancet 380: 1606–1619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

