Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis
- PMID: 31694925
- PMCID: PMC6905432
- DOI: 10.1126/scitranslmed.aax0481
Platelet regulation of myeloid suppressor of cytokine signaling 3 accelerates atherosclerosis
Abstract
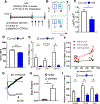
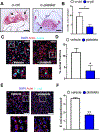
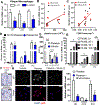
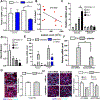
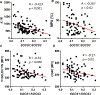
Platelets are best known as mediators of hemostasis and thrombosis; however, their inflammatory effector properties are increasingly recognized. Atherosclerosis, a chronic vascular inflammatory disease, represents the interplay between lipid deposition in the artery wall and unresolved inflammation. Here, we reveal that platelets induce monocyte migration and recruitment into atherosclerotic plaques, resulting in plaque platelet-macrophage aggregates. In Ldlr -/- mice fed a Western diet, platelet depletion decreased plaque size and necrotic area and attenuated macrophage accumulation. Platelets drive atherogenesis by skewing plaque macrophages to an inflammatory phenotype, increasing myeloid suppressor of cytokine signaling 3 (SOCS3) expression and reducing the Socs1:Socs3 ratio. Platelet-induced Socs3 expression regulates plaque macrophage reprogramming by promoting inflammatory cytokine production (Il6, Il1b, and Tnfa) and impairing phagocytic capacity, dysfunctions that contribute to unresolved inflammation and sustained plaque growth. Translating our data to humans with cardiovascular disease, we found that women with, versus without, myocardial infarction have up-regulation of SOCS3, lower SOCS1:SOCS3, and increased monocyte-platelet aggregate. A second cohort of patients with lower extremity atherosclerosis demonstrated that SOCS3 and the SOCS1:SOCS3 ratio correlated with platelet activity and inflammation. Collectively, these data provide a causative link between platelet-mediated myeloid inflammation and dysfunction, SOCS3, and cardiovascular disease. Our findings define an atherogenic role of platelets and highlight how, in the absence of thrombosis, platelets contribute to inflammation.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


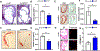
Comment in
-
Pro-inflammatory atherogenic role of platelets.Nat Rev Cardiol. 2020 Jan;17(1):6-7. doi: 10.1038/s41569-019-0312-0. Nat Rev Cardiol. 2020. PMID: 31732718 No abstract available.
-
Platelets have a dangerous hold over immune cells in cardiovascular disease.Nature. 2020 Jan;577(7790):323-324. doi: 10.1038/d41586-019-03732-9. Nature. 2020. PMID: 31937950 No abstract available.
References
-
- Gawaz M, Stellos K, and Langer HF, Platelets modulate atherogenesis and progression of atherosclerotic plaques via interaction with progenitor and dendritic cells. J Thromb Haemost, 2008. 6(2): p. 235–242. - PubMed
-
- Davi G and Patrono C, Platelet activation and atherothrombosis. N Engl J Med, 2007. 357(24): p. 2482–2494. - PubMed
-
- Wagner DD and Burger PC, Platelets in inflammation and thrombosis. Arterioscler Thromb Vasc Biol, 2003. 23(12): p. 2131–2137. - PubMed
-
- Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, and Ley K, Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med, 2003. 9(1): p. 61–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

