Genetic Diversity of Collaborative Cross Mice Controls Viral Replication, Clinical Severity, and Brain Pathology Induced by Zika Virus Infection, Independently of Oas1b
- PMID: 31694939
- PMCID: PMC7000970
- DOI: 10.1128/JVI.01034-19
Genetic Diversity of Collaborative Cross Mice Controls Viral Replication, Clinical Severity, and Brain Pathology Induced by Zika Virus Infection, Independently of Oas1b
Abstract
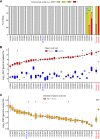
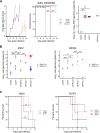
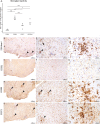
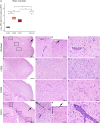
The explosive spread of Zika virus (ZIKV) has been associated with major variations in severe disease and congenital afflictions among infected populations, suggesting an influence of host genes. We investigated how genome-wide variants could impact susceptibility to ZIKV infection in mice. We first describe that the susceptibility of Ifnar1-knockout mice is largely influenced by their genetic background. We then show that Collaborative Cross (CC) mice, which exhibit a broad genetic diversity, in which the type I interferon receptor (IFNAR) was blocked by an anti-IFNAR antibody expressed phenotypes ranging from complete resistance to severe symptoms and death, with large variations in the peak and the rate of decrease in the plasma viral load, in the brain viral load, in brain histopathology, and in the viral replication rate in infected cells. The differences in susceptibility to ZIKV between CC strains correlated with the differences in susceptibility to dengue and West Nile viruses between the strains. We identified highly susceptible and resistant mouse strains as new models to investigate the mechanisms of human ZIKV disease and other flavivirus infections. Genetic analyses revealed that phenotypic variations are driven by multiple genes with small effects, reflecting the complexity of ZIKV disease susceptibility in the human population. Notably, our results rule out the possibility of a role of the Oas1b gene in the susceptibility to ZIKV. Altogether, the findings of this study emphasize the role of host genes in the pathogeny of ZIKV infection and lay the foundation for further genetic and mechanistic studies.IMPORTANCE In recent outbreaks, ZIKV has infected millions of people and induced rare but potentially severe complications, including Guillain-Barré syndrome and encephalitis in adults. While several viral sequence variants were proposed to enhance the pathogenicity of ZIKV, the influence of host genetic variants in mediating the clinical heterogeneity remains mostly unexplored. We addressed this question using a mouse panel which models the genetic diversity of the human population and a ZIKV strain from a recent clinical isolate. Through a combination of in vitro and in vivo approaches, we demonstrate that multiple host genetic variants determine viral replication in infected cells and the clinical severity, the kinetics of blood viral load, and brain pathology in mice. We describe new mouse models expressing high degrees of susceptibility or resistance to ZIKV and to other flaviviruses. These models will facilitate the identification and mechanistic characterization of host genes that influence ZIKV pathogenesis.
Keywords: Collaborative Cross; Zika; Zika virus; flavivirus; genetic diversity; host genetics; mouse model.
Copyright © 2020 American Society for Microbiology.
Figures




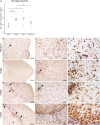
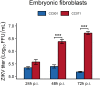
References
-
- Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. doi:10.1016/S0140-6736(16)00562-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

