Near-Atomic-Resolution Cryo-Electron Microscopy Structures of Cucumber Leaf Spot Virus and Red Clover Necrotic Mosaic Virus: Evolutionary Divergence at the Icosahedral Three-Fold Axes
- PMID: 31694952
- PMCID: PMC6955255
- DOI: 10.1128/JVI.01439-19
Near-Atomic-Resolution Cryo-Electron Microscopy Structures of Cucumber Leaf Spot Virus and Red Clover Necrotic Mosaic Virus: Evolutionary Divergence at the Icosahedral Three-Fold Axes
Abstract
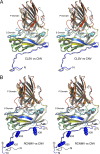
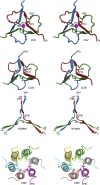
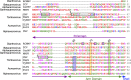
Members of the Tombusviridae family have highly similar structures, and yet there are important differences among them in host, transmission, and capsid stabilities. Viruses in the Tombusviridae family have single-stranded RNA (ssRNA) genomes with T=3 icosahedral protein shells with a maximum diameter of ∼340 Å. Each capsid protein is comprised of three domains: R (RNA binding), S (shell), and P (protruding). Between the R domain and S domain is the "arm" region that studies have shown to play a critical role in assembly. To better understand how the details of structural differences and similarities influence the Tombusviridae viral life cycles, the structures of cucumber leaf spot virus (CLSV; genus Aureusvirus) and red clover necrotic mosaic virus (RCNMV; genus Dianthovirus) were determined to resolutions of 3.2 Å and 2.9 Å, respectively, with cryo-electron microscopy and image reconstruction methods. While the shell domains had homologous structures, the stabilizing interactions at the icosahedral 3-fold axes and the R domains differed greatly. The heterogeneity in the R domains among the members of the Tombusviridae family is likely correlated with differences in the sizes and characteristics of the corresponding genomes. We propose that the changes in the R domain/RNA interactions evolved different arm domain interactions at the β-annuli. For example, RCNMV has the largest genome and it appears to have created the necessary space in the capsid by evolving the shortest R domain. The resulting loss in RNA/R domain interactions may have been compensated for by increased intersubunit β-strand interactions at the icosahedral 3-fold axes. Therefore, the R and arm domains may have coevolved to package different genomes within the conserved and rigid shell.IMPORTANCE Members of the Tombusviridae family have nearly identical shells, and yet they package genomes that range from 4.6 kb (monopartite) to 5.3 kb (bipartite) in size. To understand how this genome flexibility occurs within a rigidly conserved shell, we determined the high-resolution cryo-electron microscopy (cryo-EM) structures of cucumber leaf spot virus and red clover necrotic mosaic virus. In response to genomic size differences, it appears that the ssRNA binding (R) domain of the capsid diverged evolutionarily in order to recognize the different genomes. The next region, the "arm," seems to have also coevolved with the R domain to allow particle assembly via interactions at the icosahedral 3-fold axes. In addition, there are differences at the icosahedral 3-fold axes with regard to metal binding that are likely important for transmission and the viral life cycle.
Keywords: Tombusviridae; electron microscopy; plant viruses; virion structure.
Copyright © 2020 American Society for Microbiology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

