Brucella Exposure Risk Events in 10 Clinical Laboratories, New York City, USA, 2015 to 2017
- PMID: 31694974
- PMCID: PMC6989065
- DOI: 10.1128/JCM.01096-19
Brucella Exposure Risk Events in 10 Clinical Laboratories, New York City, USA, 2015 to 2017
Abstract
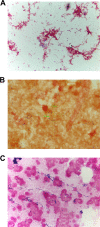
From 2015 to 2017, 11 confirmed brucellosis cases were reported in New York City, leading to 10 Brucella exposure risk events (Brucella events) in 7 clinical laboratories (CLs). Most patients had traveled to countries where brucellosis is endemic and presented with histories and findings consistent with brucellosis. CLs were not notified that specimens might yield a hazardous organism, as the clinicians did not consider brucellosis until they were notified that bacteremia with Brucella was suspected. In 3 Brucella events, the CLs did not suspect that slow-growing, small Gram-negative bacteria might be harmful. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS), which has a limited capacity to identify biological threat agents (BTAs), was used during 4 Brucella events, which accounted for 84% of exposures. In 3 of these incidents, initial staining of liquid media showed Gram-positive rods or cocci, including some cocci in chains, suggesting streptococci. Over 200 occupational exposures occurred when the unknown isolates were manipulated and/or tested on open benches, including by procedures that could generate infectious aerosols. During 3 Brucella events, the CLs examined and/or manipulated isolates in a biological safety cabinet (BSC); in each CL, the CL had previously isolated Brucella Centers for Disease Control and Prevention recommendations to prevent laboratory-acquired brucellosis (LAB) were followed; no seroconversions or LAB cases occurred. Laboratory assessments were conducted after the Brucella events to identify facility-specific risks and mitigations. With increasing MALDI-TOF MS use, CLs are well-advised to adhere strictly to safe work practices, such as handling and manipulating all slow-growing organisms in BSCs and not using MALDI-TOF MS for identification until BTAs have been ruled out.
Keywords: biosafety; brucellosis; laboratory-acquired infection; risk assessment.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Godfroid J, Cloeckaert A, Liautard J-P, Kohler S, Fretin D, Walravens K, Garin-Bastuji B, Letesson J-J. 2005. From the discovery of the Malta fever’s agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res 36:313–326. doi:10.1051/vetres:2005003. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

