The intrinsic circadian clock in podocytes controls glomerular filtration rate
- PMID: 31695128
- PMCID: PMC6838779
- DOI: 10.1038/s41598-019-52682-9
The intrinsic circadian clock in podocytes controls glomerular filtration rate
Abstract
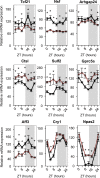
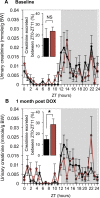
Glomerular filtration rate (GFR), or the rate of primary urine formation, is the key indicator of renal function. Studies have demonstrated that GFR exhibits significant circadian rhythmicity and, that these rhythms are disrupted in a number of pathologies. Here, we tested a hypothesis that the circadian rhythm of GFR is driven by intrinsic glomerular circadian clocks. We used mice lacking the circadian clock protein BMAL1 specifically in podocytes, highly specialized glomerular cells critically involved in the process of glomerular filtration (Bmal1lox/lox/Nphs2-rtTA/LC1 or, cKO mice). Circadian transcriptome profiling performed on isolated glomeruli from control and cKO mice revealed that the circadian clock controls expression of multiple genes encoding proteins essential for normal podocyte function. Direct assessment of glomerular filtration by inulin clearance demonstrated that circadian rhythmicity in GFR was lost in cKO mice that displayed an ultradian rhythm of GFR with 12-h periodicity. The disruption of circadian rhythmicity in GFR was paralleled by significant changes in circadian patterns of urinary creatinine, sodium, potassium and water excretion and by alteration in the diurnal pattern of plasma aldosterone levels. Collectively, these results indicate that the intrinsic circadian clock in podocytes participate in circadian rhythmicity of GFR.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

