Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence
- PMID: 31695151
- PMCID: PMC7018660
- DOI: 10.1038/s41388-019-1080-3
Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence
Erratum in
-
Correction: Nuclear receptor HNF4α performs a tumor suppressor function in prostate cancer via its induction of p21-driven cellular senescence.Oncogene. 2020 Sep;39(39):6263. doi: 10.1038/s41388-020-1290-8. Oncogene. 2020. PMID: 32820251 Free PMC article.
Abstract
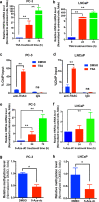
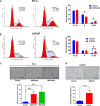
Hepatocyte nuclear factor 4α (HNF4α, NR2A1) is a highly conserved member of the nuclear receptor superfamily. Recent advances reveal that it is a key transcriptional regulator of genes, broadly involved in xenobiotic and drug metabolism and also cancers of gastrointestinal tract. However, the exact functional roles of HNF4α in prostate cancer progression are still not fully understood. In this study, we determined the functional significance of HNF4α in prostate cancer. Our results showed that HNF4α exhibited a reduced expression pattern in clinical prostate cancer tissues, prostate cancer cell lines and xenograft model of castration-relapse prostate cancer. Stable HNF4α knockdown not only could promote cell proliferation and suppress doxorubicin (Dox)-induced cellular senescence in prostate cancer cells, but also confer resistance to paclitaxel treatment and enhance colony formation capacity and in vivo tumorigenicity of prostate cancer cells. On the contrary, ectopic overexpression of HNF4α could significantly inhibit the cell proliferation of prostate cancer cells, induce cell-cycle arrest at G2/M phase and trigger the cellular senescence in prostate cancer cells by activation of p21 signal pathway in a p53-independent manner via its direct transactivation of CDKN1A. Together, our results show that HNF4α performs a tumor suppressor function in prostate cancer via a mechanism of p21-driven cellular senescence.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Loaiza N, Demaria M. Cellular senescence and tumor promotion: Is aging the key? Biochim Biophys Acta. 2016;1865:155–67. - PubMed
-
- Muller M. Cellular senescence: Molecular mechanisms, in vivo significance, and redox considerations. Antioxid Redox Signal. 2009;11:59–98. - PubMed
-
- Sharma S, Shin JS, Grimshaw M, Clarke RA, Lee CS. The senescence pathway in prostatic carcinogenesis. Pathology. 2010;42:507–11. - PubMed
-
- Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387:70–82. - PubMed
-
- Moschini M, Carroll PR, Eggener SE, Epstein JI, Graefen M, Montironi R, et al. Low-risk prostate cancer: Identification, management, and outcomes. Eur Urol. 2017;72:238–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

