Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells
- PMID: 31695360
- PMCID: PMC6707379
- DOI: 10.2147/IJN.S217245
Novel chitosan/agarose/hydroxyapatite nanocomposite scaffold for bone tissue engineering applications: comprehensive evaluation of biocompatibility and osteoinductivity with the use of osteoblasts and mesenchymal stem cells
Abstract
Background: Nanocomposites produced by reinforcement of polysaccharide matrix with nanoparticles are widely used in engineering of biomaterials. However, clinical applications of developed novel biomaterials are often limited due to their poor biocompatibility.
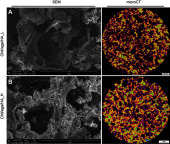
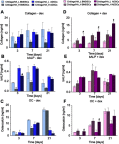
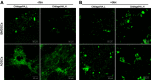
Purpose: The aim of this work was to comprehensively assess biocompatibility of highly macroporous chitosan/agarose/nanohydroxyapatite bone scaffolds produced by a novel method combining freeze-drying with a foaming agent. Within these studies, blood plasma protein adsorption, osteoblast (MC3T3-E1 Subclone 4 and hFOB 1.19) adhesion and proliferation, and osteogenic differentiation of mesenchymal stem cells derived from bone marrow and adipose tissue were determined. The obtained results were also correlated with materials' surface chemistry and wettability to explain the observed protein and cellular response.
Results: Obtained results clearly showed that the developed nanocomposite scaffolds were characterized by high biocompatibility and osteoconductivity. Importantly, the scaffolds also revealed osteoinductive properties since they have the ability to induce osteogenic differentiation (Runx2 synthesis) in undifferentiated mesenchymal stem cells. The surface of biomaterials is extremely hydrophilic, prone to protein adsorption with the highest affinity toward fibronectin binding, which allows for good osteoblast adhesion, spreading, and proliferation.
Conclusion: Produced by a novel method, macroporous nanocomposite biomaterials have great potential to be used in regenerative medicine for acceleration of the bone healing process.
Keywords: XPS; cell proliferation; cryogel; osteogenic differentiation; protein adsorption; wettability.
© 2019 Kazimierczak et al.
Conflict of interest statement
Paulina Kazimierczak and Agata Przekora report grants from NCN and Operational Program Development of Eastern Poland 2007–2013, during the conduct of the study; in addition, they have a patent, PL number P.426788, pending. Aleksandra Benko reports a grant from NCN, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures







Similar articles
-
The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro.Int J Mol Sci. 2021 Jan 23;22(3):1109. doi: 10.3390/ijms22031109. Int J Mol Sci. 2021. PMID: 33498630 Free PMC article.
-
Development of genipin-crosslinked and fucoidan-adsorbed nano-hydroxyapatite/hydroxypropyl chitosan composite scaffolds for bone tissue engineering.Int J Biol Macromol. 2019 May 1;128:973-984. doi: 10.1016/j.ijbiomac.2019.02.010. Epub 2019 Feb 8. Int J Biol Macromol. 2019. PMID: 30738901
-
Biological Response to Macroporous Chitosan-Agarose Bone Scaffolds Comprising Mg- and Zn-Doped Nano-Hydroxyapatite.Int J Mol Sci. 2019 Aug 6;20(15):3835. doi: 10.3390/ijms20153835. Int J Mol Sci. 2019. PMID: 31390753 Free PMC article.
-
Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110698. doi: 10.1016/j.msec.2020.110698. Epub 2020 Jan 29. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204012 Free PMC article. Review.
-
Chitosan composite with mesenchymal stem cells: Properties, mechanism, and its application in bone regeneration.Int J Biol Macromol. 2024 Aug;275(Pt 1):133502. doi: 10.1016/j.ijbiomac.2024.133502. Epub 2024 Jul 2. Int J Biol Macromol. 2024. PMID: 38960259 Review.
Cited by
-
Freeze-Dried Curdlan/Whey Protein Isolate-Based Biomaterial as Promising Scaffold for Matrix-Associated Autologous Chondrocyte Transplantation-A Pilot In-Vitro Study.Cells. 2022 Jan 14;11(2):282. doi: 10.3390/cells11020282. Cells. 2022. PMID: 35053397 Free PMC article.
-
A Novel Bone Substitute Based on Recombinant Type I Collagen for Reconstruction of Alveolar Cleft.Materials (Basel). 2021 Apr 29;14(9):2306. doi: 10.3390/ma14092306. Materials (Basel). 2021. PMID: 33946797 Free PMC article.
-
Swelling, Protein Adsorption, and Biocompatibility of Pectin-Chitosan Hydrogels.Gels. 2024 Jul 17;10(7):472. doi: 10.3390/gels10070472. Gels. 2024. PMID: 39057495 Free PMC article.
-
The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro.Int J Mol Sci. 2021 Jan 23;22(3):1109. doi: 10.3390/ijms22031109. Int J Mol Sci. 2021. PMID: 33498630 Free PMC article.
-
Advances in 3D-printed scaffold technologies for bone defect repair: materials, biomechanics, and clinical prospects.Biomed Eng Online. 2025 Apr 30;24(1):51. doi: 10.1186/s12938-025-01381-w. Biomed Eng Online. 2025. PMID: 40301861 Free PMC article. Review.
References
-
- Muzzarelli RAA. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr Polym. 2011;83:1433–1445. doi:10.1016/j.carbpol.2010.10.044 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

