Proarrhythmia in the p.Met207Val PITX2c-Linked Familial Atrial Fibrillation-Insights From Modeling
- PMID: 31695623
- PMCID: PMC6818469
- DOI: 10.3389/fphys.2019.01314
Proarrhythmia in the p.Met207Val PITX2c-Linked Familial Atrial Fibrillation-Insights From Modeling
Abstract
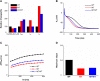
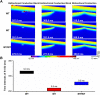
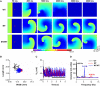
Functional analysis has shown that the p.Met207Val mutation was linked to atrial fibrillation and caused an increase in transactivation activity of PITX2c, which caused changes in mRNA synthesis related to ionic channels and intercellular electrical coupling. We assumed that these changes were quantitatively translated to the functional level. This study aimed to investigate the potential impact of the PITX2c p.Met207Val mutation on atrial electrical activity through multiscale computational models. The well-known Courtemanche-Ramirez-Nattel (CRN) model of human atrial cell action potentials (APs) was modified to incorporate experimental data on the expected p.Met207Val mutation-induced changes in ionic channel currents (I NaL , I Ks , and I Kr ) and intercellular electrical coupling. The cell models for wild-type (WT), heterozygous (Mutant/Wild type, MT/WT), and homozygous (Mutant, MT) PITX2c cases were incorporated into homogeneous multicellular 1D and 2D tissue models. Effects of this mutation-induced remodeling were quantified as changes in AP profile, AP duration (APD) restitution, conduction velocity (CV) restitution and wavelength (WL). Temporal and spatial vulnerabilities of atrial tissue to the genesis of reentry were computed. Dynamic behaviors of re-entrant excitation waves (Life span, tip trajectory and dominant frequency) in a homogeneous 2D tissue model were characterized. Our results suggest that the PITX2c p.Met207Val mutation abbreviated atrial APD and flattened APD restitution curves. It reduced atrial CV and WL that facilitated the conduction of high rate atrial excitation waves. It increased the tissue's temporal vulnerability by increasing the vulnerable window for initiating reentry and increased the tissue spatial vulnerability by reducing the substrate size necessary to sustain reentry. In the 2D models, the mutation also stabilized and accelerated re-entrant excitation waves, leading to rapid and sustained reentry. In conclusion, electrical and structural remodeling arising from the PITX2c p.Met207Val mutation may increase atrial susceptibility to arrhythmia due to shortened APD, reduced CV and increased tissue vulnerability, which, in combination, facilitate initiation and maintenance of re-entrant excitation waves.
Keywords: PITX2c; atrial fibrillation; electrical and structural remodeling; gene regulation; human atrial action potential model; modeling and simulation; single nucleotide polymorphism; transcription factors.
Copyright © 2019 Bai, Lu, Lo, Zhao and Zhang.
Figures




Similar articles
-
Pro-arrhythmogenic effects of the S140G KCNQ1 mutation in human atrial fibrillation - insights from modelling.J Physiol. 2012 Sep 15;590(18):4501-14. doi: 10.1113/jphysiol.2012.229146. Epub 2012 Apr 16. J Physiol. 2012. PMID: 22508963 Free PMC article.
-
PITX2 upregulation increases the risk of chronic atrial fibrillation in a dose-dependent manner by modulating IKs and ICaL -insights from human atrial modelling.Ann Transl Med. 2020 Mar;8(5):191. doi: 10.21037/atm.2020.01.90. Ann Transl Med. 2020. PMID: 32309338 Free PMC article.
-
Atrial proarrhythmia due to increased inward rectifier current (I(K1)) arising from KCNJ2 mutation--a simulation study.Prog Biophys Mol Biol. 2008 Oct-Nov;98(2-3):186-97. doi: 10.1016/j.pbiomolbio.2008.10.010. Epub 2008 Nov 9. Prog Biophys Mol Biol. 2008. PMID: 19041665
-
Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria.J Physiol. 2013 Sep 1;591(17):4249-72. doi: 10.1113/jphysiol.2013.254987. Epub 2013 Jun 3. J Physiol. 2013. PMID: 23732649 Free PMC article.
-
Biomimetic Cardiac Tissue Models for In Vitro Arrhythmia Studies.Biomimetics (Basel). 2023 Oct 14;8(6):487. doi: 10.3390/biomimetics8060487. Biomimetics (Basel). 2023. PMID: 37887618 Free PMC article. Review.
Cited by
-
Computational modeling of cardiac electrophysiology and arrhythmogenesis: toward clinical translation.Physiol Rev. 2024 Jul 1;104(3):1265-1333. doi: 10.1152/physrev.00017.2023. Epub 2023 Dec 28. Physiol Rev. 2024. PMID: 38153307 Free PMC article. Review.
-
In silico study of the effects of anti-arrhythmic drug treatment on sinoatrial node function for patients with atrial fibrillation.Sci Rep. 2020 Jan 15;10(1):305. doi: 10.1038/s41598-019-57246-5. Sci Rep. 2020. PMID: 31941982 Free PMC article.
-
In Silico Assessment of Class I Antiarrhythmic Drug Effects on Pitx2-Induced Atrial Fibrillation: Insights from Populations of Electrophysiological Models of Human Atrial Cells and Tissues.Int J Mol Sci. 2021 Jan 27;22(3):1265. doi: 10.3390/ijms22031265. Int J Mol Sci. 2021. PMID: 33514068 Free PMC article.
-
Mechanisms underlying pro-arrhythmic abnormalities arising from Pitx2-induced electrical remodelling: an in silico intersubject variability study.Ann Transl Med. 2021 Jan;9(2):106. doi: 10.21037/atm-20-5660. Ann Transl Med. 2021. PMID: 33569408 Free PMC article.
-
Computational Analysis of Mapping Catheter Geometry and Contact Quality Effects on Rotor Detection in Atrial Fibrillation.Front Physiol. 2021 Dec 9;12:732161. doi: 10.3389/fphys.2021.732161. eCollection 2021. Front Physiol. 2021. PMID: 34955872 Free PMC article.
References
-
- Allessie M. A., Bonke F. I., Schopman F. J. (1977). Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The “leading circle” concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ. Res. 41, 9–18. 10.1161/01.RES.41.1.9 - DOI - PubMed
-
- Bai J., Wang K., Liu Y., Li Y., Liang C., Luo G., et al. . (2017a). Computational cardiac modeling reveals mechanisms of ventricular arrhythmogenesis in long QT syndrome type 8: CACNA1C R858H mutation linked to ventricular fibrillation. Front. Physiol. 8:771. 10.3389/fphys.2017.00771 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

