Upregulation of LncRNA Malat1 Induced Proliferation and Migration of Airway Smooth Muscle Cells via miR-150-eIF4E/Akt Signaling
- PMID: 31695627
- PMCID: PMC6817469
- DOI: 10.3389/fphys.2019.01337
Upregulation of LncRNA Malat1 Induced Proliferation and Migration of Airway Smooth Muscle Cells via miR-150-eIF4E/Akt Signaling
Abstract
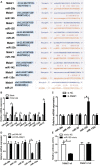
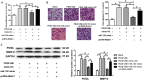
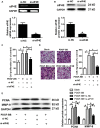
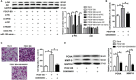
The increased proliferation and migration of airway smooth muscle cells (ASMCs) are critical processes in the formation of airway remodeling in asthma. Long non-coding RNAs (lncRNAs) have emerged as key mediators of diverse physiological and pathological processes, and are involved in the pathogenesis of various diseases, including asthma. LncRNA Malat1 has been widely reported to regulate the proliferation and migration of multiple cell types and be involved in the pathogenesis of various human diseases. However, it remains unknown whether Malat1 regulates ASMC proliferation and migration. Here, we explored the function of Malat1 in ASMC proliferation and migration in vitro stimulated by platelet-derived growth factor BB (PDGF-BB), and the underlying molecular mechanism involved. The results showed that Malat1 was significantly upregulated in ASMCs treated with PDGF-BB, and knockdown of Malat1 effectively inhibited ASMC proliferation and migration induced by PDGF-BB. Our data also showed that miR-150 was a target of Malat1 in ASMCs, and inhibited PDGF-BB-induced ASMC proliferation and migration, whereas the inhibition effect was effectively reversed by Malat1 overexpression. Additionally, translation initiation factor 4E (eIF4E), an important regulator of Akt signaling, was identified to be a target of miR-150, and both eIF4E knockdown and Akt inhibitor GSK690693 inhibited PDGF-BB-induced ASMC proliferation and migration. Collectively, these data indicate that Malat1, as a competing endogenous RNA (ceRNA) for miR-150, derepresses eIF4E expression and activates Akt signaling, thereby being involved in PDGF-BB-induced ASMC proliferation and migration. These findings suggest that Malat1 knockdown may present a new target to limit airway remodeling in asthma.
Keywords: Akt signaling; Malat1; asthma; eIF4E; miR-150.
Copyright © 2019 Lin, Li, Hao, Zhang, Zhao and Han.
Figures


Similar articles
-
Galectin-1 inhibits PDGF-BB-induced proliferation and migration of airway smooth muscle cells through the inactivation of PI3K/Akt signaling pathway.Biosci Rep. 2020 Jun 26;40(6):BSR20193899. doi: 10.1042/BSR20193899. Biosci Rep. 2020. PMID: 32495835 Free PMC article.
-
The lncRNA PVT1/miR-590-5p/FSTL1 axis modulates the proliferation and migration of airway smooth muscle cells in asthma.Autoimmunity. 2021 May;54(3):138-147. doi: 10.1080/08916934.2021.1897977. Epub 2021 Apr 7. Autoimmunity. 2021. PMID: 33825599
-
TIPE2 inhibits PDGF-BB-induced phenotype switching in airway smooth muscle cells through the PI3K/Akt signaling pathway.Respir Res. 2021 Aug 26;22(1):238. doi: 10.1186/s12931-021-01826-5. Respir Res. 2021. PMID: 34446024 Free PMC article.
-
MicroRNA-638 inhibits human airway smooth muscle cell proliferation and migration through targeting cyclin D1 and NOR1.J Cell Physiol. 2018 Jan;234(1):369-381. doi: 10.1002/jcp.26930. Epub 2018 Aug 4. J Cell Physiol. 2018. PMID: 30076719 Free PMC article. Review.
-
Role of Platelet-Derived Growth Factor (PDGF) in Asthma as an Immunoregulatory Factor Mediating Airway Remodeling and Possible Pharmacological Target.Front Pharmacol. 2020 Feb 14;11:47. doi: 10.3389/fphar.2020.00047. eCollection 2020. Front Pharmacol. 2020. PMID: 32116722 Free PMC article. Review.
Cited by
-
Single-Cell RNA-Seq Analysis Reveals Lung Epithelial Cell Type-Specific Responses to HDM and Regulation by Tet1.Genes (Basel). 2022 May 14;13(5):880. doi: 10.3390/genes13050880. Genes (Basel). 2022. PMID: 35627266 Free PMC article.
-
Gene expression profiles and bioinformatics analysis in lung samples from ovalbumin-induced asthmatic mice.BMC Pulm Med. 2023 Feb 2;23(1):50. doi: 10.1186/s12890-023-02306-w. BMC Pulm Med. 2023. PMID: 36726128 Free PMC article.
-
MicroRNA-30b-5p promotes the proliferation and migration of human airway smooth muscle cells induced by platelet-derived growth factor by targeting phosphatase and tensin homolog deleted on chromosome ten.Bioengineered. 2021 Dec;12(1):3662-3673. doi: 10.1080/21655979.2021.1950401. Bioengineered. 2021. PMID: 34251961 Free PMC article.
-
Long non-coding RNA MALAT1 exacerbates acute respiratory distress syndrome by upregulating ICAM-1 expression via microRNA-150-5p downregulation.Aging (Albany NY). 2020 Apr 21;12(8):6570-6585. doi: 10.18632/aging.102953. Epub 2020 Apr 21. Aging (Albany NY). 2020. PMID: 32315984 Free PMC article.
-
Long non-coding RNA TUG1 accelerates abnormal growth of airway smooth muscle cells in asthma by targeting the miR-138-5p/E2F3 axis.Exp Ther Med. 2021 Nov;22(5):1229. doi: 10.3892/etm.2021.10663. Epub 2021 Aug 27. Exp Ther Med. 2021. PMID: 34539825 Free PMC article.
References
-
- Austin P. J., Tsitsiou E., Boardman C., Jones S. W., Lindsay M. A., Adcock I. M., et al. . (2017). Transcriptional profiling identifies the long noncoding RNA plasmacytoma variant translocation (PVT1) as a novel regulator of the asthmatic phenotype in human airway smooth muscle. J. Allergy Clin. Immunol. 139, 780–789. 10.1016/j.jaci.2016.06.014, PMID: - DOI - PMC - PubMed
-
- Bartel S., Carraro G., Alessandrini F., Krauss-Etschmann S., Ricciardolo F. L., Bellusci S. (2018). miR-142-3p is associated with aberrant Wingless/Integrase I (WNT) signaling during airway remodeling in asthma. Am. J. Phys. Lung Cell. Mol. Phys. 315, 328–333. 10.1152/ajplung.00113.2018, PMID: - DOI - PubMed
-
- Bousquet J., Mantzouranis E., Cruz A. A., Aït-Khaled N., Baena-Cagnani C. E., Bleecker E. R., et al. . (2010). Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization consultation on severe asthma. J. Allergy Clin. Immunol. 126, 926–938. 10.1016/j.jaci.2010.07.019, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

