NKT-Like (CD3+CD56+) Cells in Chronic Myeloid Leukemia Patients Treated With Tyrosine Kinase Inhibitors
- PMID: 31695700
- PMCID: PMC6817724
- DOI: 10.3389/fimmu.2019.02493
NKT-Like (CD3+CD56+) Cells in Chronic Myeloid Leukemia Patients Treated With Tyrosine Kinase Inhibitors
Abstract
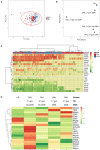
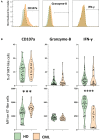
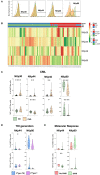
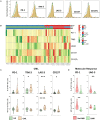
Therapy with Tyrosine Kinase Inhibitors (TKI) aiming stable deep molecular response is the gold standard to treat Chronic Myeloid Leukemia (CML). NKT-like cells (CD3+CD56+) combine characteristics of T and NK cells. The physiopathological role of these cells remains unknown although the literature refers their association with inflammation, autoimmune diseases, and cancer. Since the information regarding the role of NKT-like cells in CML is rare, we aimed at the characterization of these cells in CML patients treated with TKIs. Peripheral blood NKT-like cells from 48 CML patients and 40 healthy donors were analyzed by multiparametric flow cytometry. Functional tests consisting of co-culture with leukemic target cells (K562 cell line) were used to measure degranulation and cytokine production. Our results revealed that NKT-like cells are decreased in treated CML patients, although they present increased expression of activation markers (CD69 and HLA-DR), increased degranulation (CD107a) and impaired IFN-γ production. Significantly alterations on the expression of tumor recognition (NCRs and NKp80), and immune regulation receptors (LAG-3, TIM-3, and CD137) by NKT-like cells were observed in CML patients. Second generation TKIs increased cell activation (CD69) and decreased expression of NKp44 and NKp80 by NKT-like cells from CML patients when compared to Imatinib. CML patients that achieved deep molecular response (MR4.5) presented downregulation of NKp44 and LAG-3. Further studies are needed to clarify the role of these cells as biomarkers of therapy response and also to evaluate their value for discrimination of better candidates for sustained treatment-free remission after TKI discontinuation.
Keywords: NKT-like cells; chronic myeloid leukemia; immune checkpoints; natural cytotoxicity receptors; tyrosine kinase inhibitors.
Copyright © 2019 Almeida, Couceiro, López-Sejas, Alves, Růžičková, Tarazona, Solana, Freitas-Tavares, Santos-Rosa and Rodrigues-Santos.
Figures



References
-
- Nowell PC, Hungerford D. A minute chromosome in human chronic granulocytic leukemia. Science. (1960) 142:1497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

