Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations
- PMID: 31695764
- PMCID: PMC6831300
- DOI: 10.7150/thno.32058
Generation of human induced pluripotent stem cell-derived cardiomyocytes in 2D monolayer and scalable 3D suspension bioreactor cultures with reduced batch-to-batch variations
Abstract
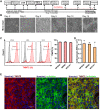
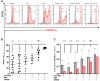
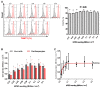
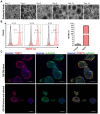
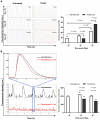
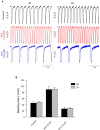
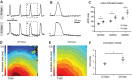
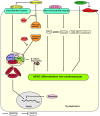
Human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) are promising candidates to treat myocardial infarction and other cardiac diseases. Such treatments require pure cardiomyocytes (CMs) in large quantities. Methods: In the present study we describe an improved protocol for production of hiPSC-CMs in which hiPSCs are first converted into mesodermal cells by stimulation of wingless (Wnt) signaling using CHIR99021, which are then further differentiated into CM progenitors by simultaneous inhibition of porcupine and tankyrase pathways using IWP2 and XAV939 under continuous supplementation of ascorbate during the entire differentiation procedure. Results: The protocol resulted in reproducible generation of >90% cardiac troponin T (TNNT2)-positive cells containing highly organized sarcomeres. In 2D monolayer cultures CM yields amounted to 0.5 million cells per cm2 growth area, and on average 72 million cells per 100 mL bioreactor suspension culture without continuous perfusion. The differentiation efficiency was hardly affected by the initial seeding density of undifferentiated hiPSCs. Furthermore, batch-to-batch variations were reduced by combinatorial use of ascorbate, IWP2, and XAV939. Conclusion: Combined inhibition of porcupine and tankyrase sub-pathways of Wnt signaling and continuous ascorbate supplementation, enable robust and efficient production of hiPSC-CMs.
Keywords: Human induced pluripotent stem cells; Wnt signaling; ascorbate; cardiomyocytes; differentiation; hiPSCs; iPS cells; regenerative medicine, bioreactor suspension culture; robust method.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Calvert JW. Chapter 5 - Ischemic Heart Disease and its Consequences A2 - Willis, Monte S. In: Homeister JW, Stone JR, editors. Cellular and Molecular Pathobiology of Cardiovascular Disease. San Diego: Academic Press; 2014. p. 79-100.
-
- Sinnecker D, Dirschinger RJ, Goedel A, Moretti A, Lipp P, Laugwitz K-L. Induced Pluripotent Stem Cells in Cardiovascular Research. In: Nilius B, Amara SG, Gudermann T, Jahn R, Lill R, Offermanns S, et al., editors. Reviews of Physiology, Biochemistry and Pharmacology, Vol 163. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. p. 1-26. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–72. - PubMed
-
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

