Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment
- PMID: 31696114
- PMCID: PMC6817476
- DOI: 10.3389/fbioe.2019.00293
Recent Advances in Nanomaterials-Based Chemo-Photothermal Combination Therapy for Improving Cancer Treatment
Abstract
Conventional chemotherapy for cancer treatment is usually compromised by shortcomings such as insufficient therapeutic outcome and undesired side effects. The past decade has witnessed the rapid development of combination therapy by integrating chemotherapy with hyperthermia for enhanced therapeutic efficacy. Near-infrared (NIR) light-mediated photothermal therapy, which has advantages such as great capacity of heat ablation and minimally invasive manner, has emerged as a powerful approach for cancer treatment. A variety of nanomaterials absorbing NIR light to generate heat have been developed to simultaneously act as carriers for chemotherapeutic drugs, contributing as heat trigger for drug release and/or inducing hyperthermia for synergistic effects. This review aims to summarize the recent development of advanced nanomaterials in chemo-photothermal combination therapy, including metal-, carbon-based nanomaterials and particularly organic nanomaterials. The potential challenges and perspectives for the future development of nanomaterials-based chemo-photothermal therapy were also discussed.
Keywords: NIR responsive; cancer; chemo-photothermal therapy; nanomaterials; synergistic effect.
Copyright © 2019 Li, Chen, Yang, Yu, Zhang, Zhu, Yu, Ouyang, Xie, Zhao and Li.
Figures




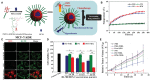
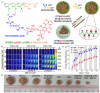
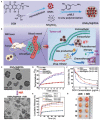
References
-
- Ardekani S. M., Dehghani A., Hassan M., Kianinia M., Aharonovich I., Gomes V. G. (2017). Two-photon excitation triggers combined chemo-photothermal therapy via doped carbon nanohybrid dots for effective breast cancer treatment. Chem. Eng. J. 330, 651–662. 10.1016/j.cej.2017.07.165 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

