Computational and cellular studies reveal structural destabilization and degradation of MLH1 variants in Lynch syndrome
- PMID: 31697235
- PMCID: PMC6837844
- DOI: 10.7554/eLife.49138
Computational and cellular studies reveal structural destabilization and degradation of MLH1 variants in Lynch syndrome
Abstract
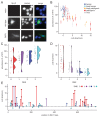
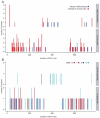
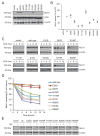
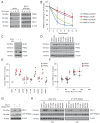
Defective mismatch repair leads to increased mutation rates, and germline loss-of-function variants in the repair component MLH1 cause the hereditary cancer predisposition disorder known as Lynch syndrome. Early diagnosis is important, but complicated by many variants being of unknown significance. Here we show that a majority of the disease-linked MLH1 variants we studied are present at reduced cellular levels. We show that destabilized MLH1 variants are targeted for chaperone-assisted proteasomal degradation, resulting also in degradation of co-factors PMS1 and PMS2. In silico saturation mutagenesis and computational predictions of thermodynamic stability of MLH1 missense variants revealed a correlation between structural destabilization, reduced steady-state levels and loss-of-function. Thus, we suggest that loss of stability and cellular degradation is an important mechanism underlying many MLH1 variants in Lynch syndrome. Combined with analyses of conservation, the thermodynamic stability predictions separate disease-linked from benign MLH1 variants, and therefore hold potential for Lynch syndrome diagnostics.
Keywords: cancer biology; chaperone; computational biology; human; proteasome; protein misfolding; protein quality control; systems biology.
© 2019, Abildgaard et al.
Conflict of interest statement
AA, AS, SN, KS, EP, AS, EH, IB, AG, MT, CI, KL, RH No competing interests declared
Figures




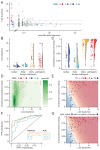
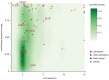
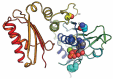

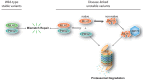
References
-
- Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin JP, Järvinen HJ. Cancer risk in mutation carriers of DNA-mismatch-repair genes. International Journal of Cancer. 1999;81:214–218. doi: 10.1002/(SICI)1097-0215(19990412)81:2<214::AID-IJC8>3.0.CO;2-L. - DOI - PubMed
-
- Andersen SD, Liberti SE, Lützen A, Drost M, Bernstein I, Nilbert M, Dominguez M, Nyström M, Hansen TV, Christoffersen JW, Jäger AC, de Wind N, Nielsen FC, Tørring PM, Rasmussen LJ. Functional characterization of MLH1 missense variants identified in lynch syndrome patients. Human Mutation. 2012;33:1647–1655. doi: 10.1002/humu.22153. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- PRISM Challenge Program/Novo Nordisk Foundation/International
- Young Investigator Award, NNF15OC0016662/Novo Nordisk Foundation/International
- Project Grant/The A.P. Møller Foundation/International
- Project Grant/Danish Cancer Society/International
- Project Grant/Danish Council for Independent Research/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

