Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program
- PMID: 31697365
- PMCID: PMC6865234
- DOI: 10.1001/jamaoncol.2019.4728
Communication With Older Patients With Cancer Using Geriatric Assessment: A Cluster-Randomized Clinical Trial From the National Cancer Institute Community Oncology Research Program
Abstract
Importance: Older patients with cancer and their caregivers worry about the effects of cancer treatment on aging-related domains (eg, function and cognition). Quality conversations with oncologists about aging-related concerns could improve patient-centered outcomes. A geriatric assessment (GA) can capture evidence-based aging-related conditions associated with poor clinical outcomes (eg, toxic effects) for older patients with cancer.
Objective: To determine whether providing a GA summary and GA-guided recommendations to oncologists can improve communication about aging-related concerns.
Design, setting, and participants: This cluster-randomized clinical trial enrolled 541 participants from 31 community oncology practices within the University of Rochester National Cancer Institute Community Oncology Research Program from October 29, 2014, to April 28, 2017. Patients were aged 70 years or older with an advanced solid malignant tumor or lymphoma who had at least 1 impaired GA domain; patients chose 1 caregiver to participate. The primary outcome was assessed on an intent-to-treat basis.
Interventions: Oncology practices were randomized to receive either a tailored GA summary with recommendations for each enrolled patient (intervention) or alerts only for patients meeting criteria for depression or cognitive impairment (usual care).
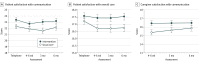
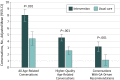
Main outcomes and measures: The predetermined primary outcome was patient satisfaction with communication about aging-related concerns (modified Health Care Climate Questionnaire [score range, 0-28; higher scores indicate greater satisfaction]), measured after the first oncology visit after the GA. Secondary outcomes included the number of aging-related concerns discussed during the visit (from content analysis of audiorecordings), quality of life (measured with the Functional Assessment of Cancer Therapy scale for patients and the 12-Item Short Form Health Survey for caregivers), and caregiver satisfaction with communication about aging-related patient concerns.
Results: A total of 541 eligible patients (264 women, 276 men, and 1 patient did not provide data; mean [SD] age, 76.6 [5.2] years) and 414 caregivers (310 women, 101 men, and 3 caregivers did not provide data; mean age, 66.5 [12.5] years) were enrolled. Patients in the intervention group were more satisfied after the visit with communication about aging-related concerns (difference in mean score, 1.09 points; 95% CI, 0.05-2.13 points; P = .04); satisfaction with communication about aging-related concerns remained higher in the intervention group over 6 months (difference in mean score, 1.10; 95% CI, 0.04-2.16; P = .04). There were more aging-related conversations in the intervention group's visits (difference, 3.59; 95% CI, 2.22-4.95; P < .001). Caregivers in the intervention group were more satisfied with communication after the visit (difference, 1.05; 95% CI, 0.12-1.98; P = .03). Quality of life outcomes did not differ between groups.
Conclusions and relevance: Including GA in oncology clinical visits for older adults with advanced cancer improves patient-centered and caregiver-centered communication about aging-related concerns.
Trial registration: ClinicalTrials.gov identifier: NCT02107443.
Conflict of interest statement
Figures
Comment in
-
Expanding the Scope of Geriatric Assessment for the Management of Cancer in Older Adults.JAMA Oncol. 2020 Feb 1;6(2):204-205. doi: 10.1001/jamaoncol.2019.4708. JAMA Oncol. 2020. PMID: 31697305 No abstract available.
References
-
- Paladino J, Bernacki R, Neville BA, et al. . Evaluating an intervention to improve communication between oncology clinicians and patients with life-limiting cancer: a cluster randomized clinical trial of the Serious Illness Care Program. JAMA Oncol. 2019;5(6):801-809. doi:10.1001/jamaoncol.2019.0292 - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

