Effectiveness of four types of neuraminidase inhibitors approved in Japan for the treatment of influenza
- PMID: 31697721
- PMCID: PMC6837752
- DOI: 10.1371/journal.pone.0224683
Effectiveness of four types of neuraminidase inhibitors approved in Japan for the treatment of influenza
Abstract
Background: Neuraminidase inhibitors (NAIs) effectively treat influenza. The clinical effectiveness of four NAIs (oseltamivir, zanamivir, laninamivir, and peramivir) was evaluated against influenza A/H1N1pdm09, A/H3N2, and B viruses. Additionally, fever duration in patients infected with oseltamivir-resistant influenza A/H1N1pdm09 with the H275Y mutation was evaluated.
Methods: Patients aged <20 years who visited outpatient clinics in Japan with influenza-like illnesses were enrolled during 4 influenza seasons from 2012/2013 to 2015/2016. After obtaining informed consent, patients who tested positive for influenza with rapid tests received one of the four NAIs. Patients recorded their body temperature daily for 8 days from the first visit. The influenza strain was identified using real-time polymerase chain reaction. Univariate and multivariable analyses were used to evaluate factors influencing fever duration. In children aged ≤5 years treated with oseltamivir, fever duration in oseltamivir-resistant A/H1N1pdm09-infected patients was compared to that in oseltamivir-sensitive A/H1N1pdm09-infected patients.
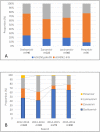
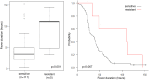
Results: Of the 1,368 patients analyzed, 297 (21.7%), 683 (49.9%), and 388 (28.4%) were infected with influenza A/H1N1pdm09, A/H3N2, and B, respectively. In multivariable analysis factors associated with significantly prolonged fever duration included: treatment with laninamivir (hazard ratio [HR]: 0.78, p = 0.006, compared to oseltamivir), influenza B (HR: 0.58, p<0.001, compared to influenza A/H1N1pdm09), and a higher body temperature at the clinic visit (HR: 0.87 per degree Celsius, p<0.001). Increasing age was associated with a significantly shorter duration of fever (HR: 1.31 for 6-9 years old, p<0.001; and HR: 1.65 for 10-19 years old, p<0.001, respectively, compared to 0-5 years old). Following treatment with oseltamivir, fever duration was significantly longer for oseltamivir-resistant A/H1N1pdm09-infected patients (n = 5) than for oseltamivir-sensitive A/H1N1pdm09 infected patients (n = 111) (mean, 89 versus 40 hours, p<0.001).
Conclusions: Our results revealed characteristic information on the effectiveness of the four NAIs and also on oseltamivir-resistant viruses that may affect patients' clinical care.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: RS received research grants from Shionogi & Co., LTD., Daiichi Sankyo Co., LTD., Chugai Pharmaceutical Co., Ltd., and GlaxoSmithKline K.K. This does not alter our adherence to PLOS ONE policies on sharing data materials.
Figures



References
-
- Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hirotsu N, et al. A comparison of the effectiveness of oseltamivir for the treatment of influenza A and influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis. 2006;43: 439–444. Epub 2006/07/14. 10.1086/505868 . - DOI - PubMed
-
- Sato M, Saito R, Sato I, Tanabe N, Shobugawa Y, Sasaki A, et al. Effectiveness of oseltamivir treatment among children with influenza A or B virus infections during four successive winters in Niigata City, Japan. Tohoku J Exp Med. 2008;214: 113–120. Epub 2008/02/21. 10.1620/tjem.214.113 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

