Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death
- PMID: 31697817
- PMCID: PMC6933290
- DOI: 10.1182/blood.2019000899
Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death
Erratum in
-
Fustolo-Gunnink SF, Fijnvandraat K, van Klaveren D, et al; PlaNeT-2 MATISSE Collaborators. Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood. 2019;134(26):2354-2360.Blood. 2020 Jun 11;135(24):2199. doi: 10.1182/blood.2020006498. Blood. 2020. PMID: 32526023 Free PMC article. No abstract available.
Abstract
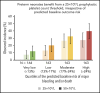
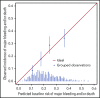
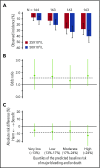
The Platelets for Neonatal Thrombocytopenia (PlaNeT-2) trial reported an unexpected overall benefit of a prophylactic platelet transfusion threshold of 25 × 109/L compared with 50 × 109/L for major bleeding and/or mortality in preterm neonates (7% absolute-risk reduction). However, some neonates in the trial may have experienced little benefit or even harm from the 25 × 109/L threshold. We wanted to assess this heterogeneity of treatment effect in the PlaNet-2 trial, to investigate whether all preterm neonates benefit from the low threshold. We developed a multivariate logistic regression model in the PlaNet-2 data to predict baseline risk of major bleeding and/or mortality for all 653 neonates. We then ranked the neonates based on their predicted baseline risk and categorized them into 4 risk quartiles. Within these quartiles, we assessed absolute-risk difference between the 50 × 109/L- and 25 × 109/L-threshold groups. A total of 146 neonates died or developed major bleeding. The internally validated C-statistic of the model was 0.63 (95% confidence interval, 0.58-0.68). The 25 × 109/L threshold was associated with absolute-risk reduction in all risk groups, varying from 4.9% in the lowest risk group to 12.3% in the highest risk group. These results suggest that a 25 × 109/L prophylactic platelet count threshold can be adopted in all preterm neonates, irrespective of predicted baseline outcome risk. Future studies are needed to improve the predictive accuracy of the baseline risk model. This trial was registered at www.isrctn.com as #ISRCTN87736839.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Fustolo-Gunnink SF, Huisman EJ, van der Bom JG, et al. . Are thrombocytopenia and platelet transfusions associated with major bleeding in preterm neonates? A systematic review. Blood Rev. 2019;36:1-9. - PubMed
-
- Stanworth SJ, Clarke P, Watts T, et al. ; Platelets and Neonatal Transfusion Study Group . Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009;124(5):e826-e834. - PubMed
-
- Curley A, Stanworth SJ, Willoughby K, et al. ; PlaNeT2 MATISSE Collaborators . Randomized Trial of Platelet-Transfusion Thresholds in Neonates. N Engl J Med. 2019;380(3):242-251. - PubMed
-
- Ioannidis JPA, Lau J. The impact of high-risk patients on the results of clinical trials. J Clin Epidemiol. 1997;50(10):1089-1098. - PubMed

